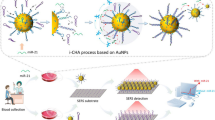
Abstract
A “turn-on” aptasensor for label-free and cell-free EpCAM detection was constructed by employing magnetic α-Fe2O3/Fe3O4@Au nanocomposites as a matrix for signal amplification and double-stranded complex (SH-DNA/Apt probes) immobilization through Au-S binding. α-Fe2O3/Fe3O4@Au could be efficiently assembled into uniform and stable self-assembly films via magnetic-induced self-assembly technique on a magnetic glassy carbon electrode (MGCE). The effectiveness of the platform for EpCAM detection was confirmed through differential pulse voltammetry (DPV). Under optimized conditions, the platform exhibited excellent specificity for EpCAM, and a strong linear correlation was observed between the current and the logarithm of EpCAM protein concentration in the range 1 pg/mL–1000 pg/mL (R2 = 0.9964), with a limit of detection (LOD) of 0.27 pg/mL. Furthermore, the developed platform demonstrated good stability during a 14-day storage test, with fluctuations remaining below 93.33% of the initial current value. Promising results were obtained when detecting EpCAM in spiked serum samples, suggesting its potential as a point-of-care (POC) testing.
Graphical Abstract






Similar content being viewed by others
References
Maetzel D, Denzel S, Mack B et al (2009) Nuclear signalling by tumour-associated antigen EpCAM. Nat Cell Biol 11:162–171. https://doi.org/10.1038/ncb1824
Herlyn M, Steplewski Z, Herlyn D, Koprowski H (1979) Colorectal carcinoma-specific antigen: detection by means of monoclonal antibodies. Proc Natl Acad Sci 76:1438–1442. https://doi.org/10.1073/pnas.76.3.1438
Baeuerle PA, Gires O (2007) EpCAM (CD326) finding its role in cancer. Br J Cancer 96:417–423. https://doi.org/10.1038/sj.bjc.6603494
Patriarca C, Macchi RM, Marschner AK, Mellstedt H (2012) Epithelial cell adhesion molecule expression (CD326) in cancer: a short review. Cancer Treat Rev 38:68–75. https://doi.org/10.1016/j.ctrv.2011.04.002
Hase T, Sato M, Yoshida K et al (2011) Pivotal role of epithelial cell adhesion molecule in the survival of lung cancer cells. Cancer Sci 102:1493–1500. https://doi.org/10.1111/j.1349-7006.2011.01973.x
Kimura H, Kato H, Faried A et al (2007) Prognostic significance of EpCAM expression in human esophageal cancer. Int J Oncol 30:171–179. https://doi.org/10.3892/ijo.30.1.171
Zhao X, Zhao R, Feng Y et al (2023) The roles EpCAM plays to enhance the malignancy of gastric cancer. J Cancer Res Clin Oncol. https://doi.org/10.1007/s00432-023-04767-2
Osta WA, Chen Y, Mikhitarian K et al (2004) EpCAM is overexpressed in breast cancer and is a potential target for breast cancer gene therapy. Cancer Res 64:5818–5824. https://doi.org/10.1158/0008-5472.CAN-04-0754
Bellone S, Siegel ER, Cocco E et al (2009) Overexpression of epithelial cell adhesion molecule in primary, metastatic, and recurrent/chemotherapy-resistant epithelial ovarian cancer: implications for epithelial cell adhesion molecule-specific immunotherapy. Int J Gynecol Cancer 19:860–866. https://doi.org/10.1111/IGC.0b013e3181a8331f
Ni J, Cozzi PJ, Duan W et al (2012) Role of the EpCAM (CD326) in prostate cancer metastasis and progression. Cancer Metastasis Rev 31:779–791. https://doi.org/10.1007/s10555-012-9389-1
Went P, Vasei M, Bubendorf L et al (2006) Frequent high-level expression of the immunotherapeutic target Ep-CAM in colon, stomach, prostate and lung cancers. Br J Cancer 94:128–135. https://doi.org/10.1038/sj.bjc.6602924
Diaz-Arias AA, Loy TS, Bickel JT, Chapman RK (1993) Utility of BER-EP4 in the diagnosis of adenocarcinoma in effusions: an immunocytochemical study of 232 cases. Diagn Cytopathol 9:516–521. https://doi.org/10.1002/dc.2840090509
Shi J, Lyu J, Tian F, Yang M (2017) A fluorescence turn-on biosensor based on graphene quantum dots (GQDs) and molybdenum disulfide (MoS2) nanosheets for epithelial cell adhesion molecule (EpCAM) detection. Biosens Bioelectron 93:182–188. https://doi.org/10.1016/j.bios.2016.09.012
Ortega FG, Fernández-Baldo MA, Serrano MJ et al (2015) Epithelial cancer biomarker EpCAM determination in peripheral blood samples using a microfluidic immunosensor based in silver nanoparticles as platform. Sens Actuators B 221:248–256. https://doi.org/10.1016/j.snb.2015.06.066
Shiddiky MJA, Rauf S, Kithva PH, Trau M (2012) Graphene/quantum dot bionanoconjugates as signal amplifiers in stripping voltammetric detection of EpCAM biomarkers. Biosens Bioelectron 35:251–257. https://doi.org/10.1016/j.bios.2012.02.057
Arya SK, Wang KY, Wong CC, Rahman ARA (2013) Anti-EpCAM modified LC-SPDP monolayer on gold microelectrode based electrochemical biosensor for MCF-7 cells detection. Biosens Bioelectron 41:446–451. https://doi.org/10.1016/j.bios.2012.09.006
Jalil O, Pandey CM, Kumar D (2021) Highly sensitive electrochemical detection of cancer biomarker based on anti-EpCAM conjugated molybdenum disulfide grafted reduced graphene oxide nanohybrid. Bioelectrochemistry 138:107733. https://doi.org/10.1016/j.bioelechem.2020.107733
Sweety KD (2023) Electrochemical immunosensor based on titanium dioxide grafted MXene for EpCAM antigen detection. J Colloid Interface Sci 652:549–556. https://doi.org/10.1016/j.jcis.2023.08.099
Tan W, Donovan MJ, Jiang J (2013) Aptamers from cell-based selection for bioanalytical applications. Chem Rev 113:2842–2862. https://doi.org/10.1021/cr300468w
Song Y, Zhu Z, An Y et al (2013) Selection of DNA aptamers against epithelial cell adhesion molecule for cancer cell imaging and circulating tumor cell capture. Anal Chem 85:4141–4149. https://doi.org/10.1021/ac400366b
Chen J, Zhuang X, Zheng J et al (2021) Aptamer-based cell-free detection system to detect target protein. Synth Syst Biotechnol 6:209–215. https://doi.org/10.1016/j.synbio.2021.07.004
Oliveira AEF, Pereira AC, Ferreira LF (2023) Disposable electropolymerized molecularly imprinted electrochemical sensor for determination of breast cancer biomarker CA 15-3 in human serum samples. Talanta 252:123819. https://doi.org/10.1016/j.talanta.2022.123819
Lu Y, Luo Q, Jia X et al (2023) Multidisciplinary strategies to enhance therapeutic effects of flavonoids from Epimedii Folium: integration of herbal medicine, enzyme engineering, and nanotechnology. J Pharm Anal 13:239–254. https://doi.org/10.1016/j.jpha.2022.12.001
Bagheri Hashkavayi A, Cha BS, Hwang SH et al (2021) Highly sensitive electrochemical detection of circulating EpCAM-positive tumor cells using a dual signal amplification strategy. Sens Actuators B 343:130087. https://doi.org/10.1016/j.snb.2021.130087
Chinnapaiyan S, Das HT, Chen S-M et al (2023) CoAl2O4 nanoparticles modified carbon nanofibers as high-efficiency bifunctional electrocatalyst: an efficient electrochemical aqueous asymmetric supercapacitors and non-enzymatic electrochemical sensors. J Alloys Compd 931:167553. https://doi.org/10.1016/j.jallcom.2022.167553
Zhang Y, Ouyang H, Zhang S et al (2023) Label-free electrochemical bioplatform based on Au-modified magnetic Fe3O4/α-Fe2O3 hetero-nanorods for sensitive quantification of ovarian cancer tumor marker. Microchem J 189:108546. https://doi.org/10.1016/j.microc.2023.108546
Ni Y, Ouyang H, Yu L et al (2022) Label-free electrochemical aptasensor based on magnetic α-Fe2O3/Fe3O4 heterogeneous hollow nanorods for the detection of cancer antigen 125. Bioelectrochemistry 148:108255. https://doi.org/10.1016/j.bioelechem.2022.108255
Liu R, Pan S, Liu M et al (2021) A label-free electrochemical biosensor with magnetically induced self-assembly for the detection of CYP2C9*3 gene. Appl Surf Sci 537:147868. https://doi.org/10.1016/j.apsusc.2020.147868
Yu L, Liu M, Zhang Y et al (2022) Magnetically induced self-assembly DNAzyme electrochemical biosensor based on gold-modified α-Fe2O3/Fe3O4 heterogeneous nanoparticles for sensitive detection of Ni2+. Nanotechnology 33:095601. https://doi.org/10.1088/1361-6528/ac3b0e
Ni Y, Chen X, Ling C et al (2023) Electrochemical peptide nucleic acid functionalized α-Fe2O3/Fe3O4 nanosheets for detection of CYP2C19*2 gene. Microchim Acta 190:189. https://doi.org/10.1007/s00604-023-05781-4
Jin H, Dong J, Qi X et al (2023) A label-free impedance-based electrochemical sensor based on self-assembled dendritic DNA nanostructures for Pb2+ detection. Bioelectrochemistry 149:108312. https://doi.org/10.1016/j.bioelechem.2022.108312
Dong X, Qi S, Qin M et al (2023) A novel biomimetic network amplification strategy designed fluorescent aptasensor based on yolk-shell Fe3O4 nanomaterials for aflatoxin B1 detection. Food Chem 398:133761. https://doi.org/10.1016/j.foodchem.2022.133761
Costanzo H, Gooch J, Frascione N (2023) Nanomaterials for optical biosensors in forensic analysis. Talanta 253:123945. https://doi.org/10.1016/j.talanta.2022.123945
Liu R, Zhang Y, Deng P et al (2022) Construction of targeted delivery system for curcumin loaded on magnetic α-Fe2O3/Fe3O4 heterogeneous nanotubes and its apoptosis mechanism on MCF-7 cell. Biomater Adv 136:212783. https://doi.org/10.1016/j.bioadv.2022.212783
Zhang S, Deng P, Yu L et al (2022) Fabrication and formation mechanism of hollow-structure supermagnetic α-Fe2O3/Fe3O4 heterogeneous nanospindles. J Inorg Organomet Polym Mater 32:2492–2501. https://doi.org/10.1007/s10904-022-02328-7
Chen R, Sun Y, Huo B et al (2021) Development of Fe3O4@Au nanoparticles coupled to Au@Ag core-shell nanoparticles for the sensitive detection of zearalenone. Anal Chim Acta 1180:338888. https://doi.org/10.1016/j.aca.2021.338888
Rizalputri LN, Anshori I, Handayani M et al (2022) Facile and controllable synthesis of monodisperse gold nanoparticle bipyramid for electrochemical dopamine sensor. Nanotechnology 34:055502. https://doi.org/10.1088/1361-6528/ac9d3f
Wei J, Qileng A, Yan Y et al (2017) A novel visible-light driven photoelectrochemical immunosensor based on multi-amplification strategy for ultrasensitive detection of microcystin-LR. Anal Chim Acta 994:82–91. https://doi.org/10.1016/j.aca.2017.09.035
Yang Y, Zhang Y, Thakur A et al (2019) One-pot synthesis and characterization of ovalbumin-conjugated gold nanoparticles: a comparative study of adjuvanticity against the physical mixture of ovalbumin and gold nanoparticles. Int J Pharm 571:118704. https://doi.org/10.1016/j.ijpharm.2019.118704
Jia Y, Guo S, Han Q et al (2021) Target-triggered and controlled release plasmon-enhanced fluorescent AIE probe for conformational monitoring of insulin fibrillation. J Mater Chem B 9:5128–5135. https://doi.org/10.1039/D1TB00712B
Zhang R, Liu S, Kong F et al (2020) α-Fe2O3/BiFeO3 composites as visible-active photocatalysts and their optical response mechanism. J Phys Chem Solid 141:109329. https://doi.org/10.1016/j.jpcs.2019.109329
Ni Y, Deng P, Yin R et al (2023) Effect and mechanism of paclitaxel loaded on magnetic Fe3O4 @mSiO2-NH2-FA nanocomposites to MCF-7 cells. Drug Deliv 30:64–82. https://doi.org/10.1080/10717544.2022.2154411
Shafi A, Bano S, Sharma L et al (2022) Exploring multifunctional behaviour of g-C3N4 decorated BiVO4/Ag2CO3 hierarchical nanocomposite for simultaneous electrochemical detection of two nitroaromatic compounds and water splitting applications. Talanta 241:123257. https://doi.org/10.1016/j.talanta.2022.123257
Bravo K, Ortega FG, Messina GA et al (2017) Integrated bio-affinity nano-platform into a microfluidic immunosensor based on monoclonal bispecific trifunctional antibodies for the electrochemical determination of epithelial cancer biomarker. Clin Chim Acta 464:64–71. https://doi.org/10.1016/j.cca.2016.11.012
Fernández-Baldo MA, Ortega FG, Pereira SV et al (2016) Nanostructured platform integrated into a microfluidic immunosensor coupled to laser-induced fluorescence for the epithelial cancer biomarker determination. Microchem J 128:18–25. https://doi.org/10.1016/j.microc.2016.03.012
Jalil O, Pandey CM, Kumar D (2020) Electrochemical biosensor for the epithelial cancer biomarker EpCAM based on reduced graphene oxide modified with nanostructured titanium dioxide. Microchim Acta 187:275. https://doi.org/10.1007/s00604-020-04233-7
Zhu L, Yang B, Qian K et al (2020) Sensitive electrochemical aptasensor for detecting EpCAM with silica nanoparticles and quantum dots for signal amplification. J Electroanal Chem 856:113655. https://doi.org/10.1016/j.jelechem.2019.113655
Chen Q, Hu W, Shang B et al (2018) Ultrasensitive amperometric aptasensor for the epithelial cell adhesion molecule by using target-driven toehold-mediated DNA recycling amplification. Microchim Acta 185:1–8. https://doi.org/10.1007/s00604-018-2739-0
Zhao Y, Yao J, Wu Z et al (2022) AuPt NPs with enhanced electrochemical oxidization activity for ratiometric electrochemical aptasensor. Biosens Bioelectron 196:113733. https://doi.org/10.1016/j.bios.2021.113733
Funding
This work was supported by the Jiangsu Provincial Postgraduate Scientific Practice and Innovation Project (Grant No. SJCX21_1722).
Author information
Authors and Affiliations
Contributions
Y.Y.: methodology, experimentation, writing–original draft, data curation. H.O.: funding acquisition, investigation, validation. M.M.: experimentation, investigation. Y.Y.: conceptualization, visualization, investigation. H.Z.: resources, software, visualization. A.H.: funding acquisition, investigation, validation. R.L.: writing–review and editing, supervision.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• A label-free and cell-free electrochemical “turn-on” aptasensor was constructed for accurate detection of EpCAM.
• Magnetic α-Fe2O3/Fe3O4@Au nanocomposites achieved promising electrochemical property.
• The rapid detection of EpCAM was achieved by magnetic self-assembly and separation strategies.
• The aptasensor revealed low LOD, excellent specificity, reproducibility, and stability.
Supplementary information
ESM 1
(DOCX 816 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yue, Y., Ouyang, H., Ma, M. et al. Nucleic acid aptasensor with magnetically induced self-assembly for the detection of EpCAM glycoprotein. Microchim Acta 191, 64 (2024). https://doi.org/10.1007/s00604-023-06117-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-023-06117-y