Abstract
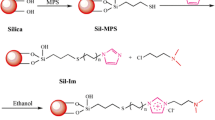
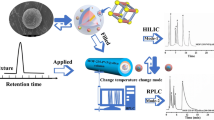
Two novel stationary phases, 1-(4-bromobutyl)-3-methylimidazolium bromide bonded chitosan modified silica and 1-(4-bromobutyl)-3-methylimidazolium bromide bonded chitosan derivatized calix[4]arene modified silica stationary phase, were synthesized using 1-(4-bromobutyl)-3-methylimidazolium bromide bonding chitosan as a polarity regulator solving the limitation of the strong hydrophobicity of calixarene in the application of hydrophilic field. The resulting materials were characterized by solid-state nuclear magnetic resonance, Fourier-transform infrared spectra, scanning electron microscopy, elemental analysis, and thermogravimetric analysis. Based on the hydrophilicity endowed by 1-(4-bromobutyl)-3-methylimidazolium bromide bonded chitosan, the retention mode of ILC-Sil and ILCC4-Sil could be effectively switched from the hydrophilic mode to a hydrophilic/hydrophobic mixed mode and could simultaneously provide various interactions with solutes, including hydrophilic, π-π, ion-exchange, inclusion, hydrophobic, and electrostatic interactions. On the basis of these interactions, successful separation and higher shape selectivity were achieved among compounds that vary in polarity under both reverse-phase and hydrophilic interactive liquid chromatography conditions. Moreover, the ILCC4-Sil was successfully applied to the determination of morphine in actual samples using solid-phase extraction and mass spectrometry. The LOD and LOQ were 15 pg/mL and 54 pg/mL, respectively. This work presents an exceptionally flexible adjustment strategy for the retention and selectivity of a silica stationary phase by tuning the modification group.
Graphical Abstract








Similar content being viewed by others
References
Alpert AJ (1990) Hydrophilic-interaction chromatography for the separation of peptides, nucleic acids and other polar compounds. J Chromatogr A 499:177–196
Qing G, Yan J, He X et al (2020) Recent advances in hydrophilic interaction liquid interaction chromatography materials for glycopeptide enrichment and glycan separation. TrAC, Trends Anal Chem 124:115570. https://doi.org/10.1016/j.trac.2019.06.020
McCalley DV (2017) Understanding and manipulating the separation in hydrophilic interaction liquid chromatography. J Chromatogr A 1523:49–71. https://doi.org/10.1016/j.chroma.2017.06.026
Salas D, Borrull F, Fontanals N, Marcé RM (2017) Hydrophilic interaction liquid chromatography coupled to mass spectrometry-based detection to determine emerging organic contaminants in environmental samples. TrAC, Trends Anal Chem 94:141–149. https://doi.org/10.1016/j.trac.2017.07.017
Hemström P, Irgum K (2006) Hydrophilic interaction chromatography. J Sep Sci 29:1784–1821. https://doi.org/10.1002/jssc.200600199
Karatapanis AE, Fiamegos YC, Stalikas CD (2011) A revisit to the retention mechanism of hydrophilic interaction liquid chromatography using model organic compounds. J Chromatogr A 1218:2871–2879. https://doi.org/10.1016/j.chroma.2011.02.069
Yuan N, Chen J, Cai T et al (2020) Glucose-based carbon dots-modified silica stationary phase for hydrophilic interaction chromatography. J Chromatogr A 1619:460930. https://doi.org/10.1016/j.chroma.2020.460930
Pazourek J (2014) Fast separation and determination of free myo-inositol by hydrophilic liquid chromatography. Carbohydr Res 391:55–60. https://doi.org/10.1016/j.carres.2014.03.010
Fan C, Chen J, Li H et al (2022) Preparation and evaluation of two silica-based hydrophilic-hydrophobic and acid-base balanced stationary phases via in-situ surface polymerization. J Chromatogr A 1667:462912. https://doi.org/10.1016/j.chroma.2022.462912
Guan F, You Y, Li X, Robinson MA (2019) A comprehensive approach to detecting multitudinous bioactive peptides in equine plasma and urine using hydrophilic interaction liquid chromatography coupled to high resolution mass spectrometry. Drug Test Anal 11:1308–1325. https://doi.org/10.1002/dta.2671
Dedvisitsakul P, Jacobsen S, Svensson B, Bunkenborg J, Christine Finnie PH (2014) Glycopeptide enrichment using a combination of ZIC-HILIC and cotton wool for exploring the glycoproteome of wheat flour albumins. J Proteome Res 13:2696–2703
Yang Y, Boysen RI, Hearn MTW (2009) Hydrophilic interaction chromatography coupled to electrospray mass spectrometry for the separation of peptides and protein digests. J Chromatogr A 1216:5518–5524. https://doi.org/10.1016/j.chroma.2009.05.085
Zhou W, Wang PG, Ogunsola OA, Kraeling MEK (2013) Rapid determination of hexapeptides by hydrophilic interaction LC-MS/MS for in vitro skin-penetration studies. Bioanalysis 5:1353–1362. https://doi.org/10.4155/bio.13.60
Douša M, Srbek J, Stránský Z et al (2014) Retention behavior of a homologous series and positional isomers of aliphatic amino acids in hydrophilic interaction chromatography. J Sep Sci 37:739–747. https://doi.org/10.1002/jssc.201301348
Noga S, Jandera P, Buszewski B (2013) Retention mechanism studies of selected amino acids and vitamin b6 on HILIC columns with evaporative light scattering detection. Chromatographia 76:929–937. https://doi.org/10.1007/s10337-013-2502-y
Iturrospe E, Da Silva KM, Talavera Andújar B et al (2021) An exploratory approach for an oriented development of an untargeted hydrophilic interaction liquid chromatography-mass spectrometry platform for polar metabolites in biological matrices. J Chromatogr A 1637:461807. https://doi.org/10.1016/j.chroma.2020.461807
Zuo H, Guo Y, Zhao W et al (2019) Controlled fabrication of silica@covalent triazine polymer core-shell spheres as a reversed-phase/hydrophilic interaction mixed-mode chromatographic stationary phase. ACS Appl Mater Interfaces 11:46149–46156. https://doi.org/10.1021/acsami.9b16438
Yaroshenko DV, Grigoriev AV, Yaroshenko IS et al (2021) Hydrophilic interaction liquid chromatography method for eremomycin determination in pre-clinical study. J Chromatogr A 1637:461750. https://doi.org/10.1016/j.chroma.2020.461750
Guo X, Zhang X, Guo Z et al (2014) Hydrophilic interaction chromatography for selective separation of isomeric saponins. J Chromatogr A 1325:121–128. https://doi.org/10.1016/j.chroma.2013.12.006
Fu Q, Guo Z, Zhang X et al (2012) Comprehensive characterization of Stevia Rebaudiana using two-dimensional reversed-phase liquid chromatography/hydrophilic interaction liquid chromatography. J Sep Sci 35:1821–1827. https://doi.org/10.1002/jssc.201101103
Wang L, Wei W, Xia Z et al (2016) Recent advances in materials for stationary phases of mixed-mode high-performance liquid chromatography. TrAC, Trends Anal Chem 80:495–506. https://doi.org/10.1016/j.trac.2016.04.001
Zhao W, Wang X, Guo J et al (2020) Evaluation of sulfonic acid functionalized covalent triazine framework as a hydrophilic-lipophilic balance/cation-exchange mixed-mode sorbent for extraction of benzimidazole fungicides in vegetables, fruits and juices. J Chromatogr A 1618:460847. https://doi.org/10.1016/j.chroma.2019.460847
Bo C, Jia Z, Dai X, Wei Y (2020) Facile preparation of polymer-brush reverse-phase/hydrophilic interaction/ion-exchange tri-mode chromatographic stationary phases by controlled polymerization of three functional monomers. J Chromatogr A 1619:460966. https://doi.org/10.1016/j.chroma.2020.460966
Liang T, Fu Q, Shen A et al (2015) Preparation and chromatographic evaluation of a newly designed steviol glycoside modified-silica stationary phase in hydrophilic interaction liquid chromatography and reversed phase liquid chromatography. J Chromatogr A 1388:110–118. https://doi.org/10.1016/j.chroma.2015.02.019
Wu Q, Hou X, Zhang X et al (2021) Amphipathic carbon quantum dots-functionalized silica stationary phase for reversed phase/hydrophilic interaction chromatography. Talanta 226:122148. https://doi.org/10.1016/j.talanta.2021.122148
Hosseini ES, Heydar KT (2021) Silica modification with 9-methylacridine and 9-undecylacridine as mixed-mode stationary phases in HPLC. Talanta 221:121445. https://doi.org/10.1016/j.talanta.2020.121445
Wu Q, Hou X, Lv H et al (2021) Synthesis of octadecylamine-derived carbon dots and application in reversed phase/hydrophilic interaction liquid chromatography. J Chromatogr A 1656:462548. https://doi.org/10.1016/j.chroma.2021.462548
Sun HF, Cui YY, Zhen CQ, Yang CX (2023) Monomer-mediated fabrication of microporous organic network@silica microsphere for reversed-phase/hydrophilic interaction mixed-mode chromatography. Talanta 251:123763. https://doi.org/10.1016/j.talanta.2022.123763
Zhang YP, Li K, Xiong LX et al (2022) “Click” preparation of a chiral macrocycle-based stationary phase for both normal-phase and reversed-phase high performance liquid chromatography enantioseparation. J Chromatogr A 1683:463551. https://doi.org/10.1016/j.chroma.2022.463551
Zhang W, Zhang Y, Zhang Y et al (2019) Tetra-proline modified calix[4]arene bonded silica gel: a novel stationary phase for hydrophilic interaction liquid chromatography. Talanta 193:56–63. https://doi.org/10.1016/j.talanta.2018.09.083
Zhang W, Zhang Y, Zhang G et al (2019) Tetra-proline modified calix[4]arene bonded silica stationary phase for simultaneous reversed-phase/hydrophilic interaction mixed-mode chromatography. J Sep Sci 1–10:1374. https://doi.org/10.1002/jssc.201800967
Lu J, Zhang W, Zhang Y et al (2014) A new stationary phase for high performance liquid chromatography: calix[4]arene derivatized chitosan bonded silica gel. J Chromatogr A 1350:61–67. https://doi.org/10.1016/j.chroma.2014.05.021
Zhao W, Hu K, Wang C et al (2012) New oxo-bridged calix[2]arene[2] triazine stationary phase for high performance liquid chromatography. J Chromatogr A 1223:72–78. https://doi.org/10.1016/j.chroma.2011.12.031
Zhao W, Wang W, Chang H et al (2012) Tetraazacalix[2]arene[2] triazine modified silica gel: a novel multi-interaction stationary phase for mixed-mode chromatography. J Chromatogr A 1251:74–81. https://doi.org/10.1016/j.chroma.2012.06.030
Zhang W, Wang F, Zhang Y et al (2019) 1,3,5-Triazine tetrad-aza cyclophanes coated silica as a novel stationary phase for high performance liquid chromatography. Sep Purif Technol 211:227–232. https://doi.org/10.1016/j.seppur.2018.09.082
Chen H-Y, Guo D, Gan Z-F et al (2019) A phenylboronate-based SERS nanoprobe for detection and imaging of intracellular peroxynitrite. Microchim Acta 186:11. https://doi.org/10.1007/s00604-018-3129-3
Ostovan A, Arabi M, Wang Y et al (2022) Greenificated molecularly imprinted materials for advanced applications. Adv Mater 34:2203154. https://doi.org/10.1002/adma.202203154
Ostovan A, Ghaedi M, Arabi M et al (2018) Hydrophilic multitemplate molecularly imprinted biopolymers based on a green synthesis strategy for determination of B-family vitamins. ACS Appl Mater Interfaces 10:4140–4150. https://doi.org/10.1021/acsami.7b17500
Arabi M, Ostovan A, Li J et al (2021) Molecular imprinting: green perspectives and strategies. Adv Mater 33:2100543. https://doi.org/10.1002/adma.202100543
Zhang L, Shen J, Zuo W, Okamoto Y (2014) Synthesis of chitosan 3,6-diphenylcarbamate-2-urea derivatives and their applications as chiral stationary phases for high-performance liquid chromatography. J Chromatogr A 1365:86–93. https://doi.org/10.1016/j.chroma.2014.09.002
Hu K, Liu J, Tang C et al (2012) Preparation, characterization and application of a new 25,27-bis-[2-(5- methylthiadiazole)thioethoxyl]-26,28-dihydroxy-para-tert-butyl calix[4]arene stationary phase for HPLC. J Sep Sci 35:239–247. https://doi.org/10.1002/jssc.201100733
Liu Y, Zou H, Haginaka J (2006) Preparation and evaluation of a novel chiral stationary phase based on covalently bonded chitosan for ligand-exchange chromatography. J Sep Sci 29:1440–1446. https://doi.org/10.1002/jssc.200600015
Deng Z, Liu J, Hu C et al (2014) Liquid chromatographic behavior of two alanine-substituted calix[4]arene-bonded silica gel stationary phases. J Sep Sci 37:3268–3275. https://doi.org/10.1002/jssc.201400366
Tanaka N, Tokuda Y, Iwaguchi K, Araki M (1982) Effect of stationary phase structure on retention and selectivity in reversed-phase liquid chromatography. J Chromatogr A 239:761–772. https://doi.org/10.1016/S0021-9673(00)82036-1
Zhao W, Chu J, Xie F et al (2017) Preparation and evaluation of pillararene bonded silica gel stationary phases for high performance liquid chromatography. J Chromatogr A 1485:44–51. https://doi.org/10.1016/j.chroma.2016.12.019
Ohyama K, Inoue Y, Kishikawa N, Kuroda N (2014) Preparation and characterization of surfactin-modified silica stationary phase for reversed-phase and hydrophilic interaction liquid chromatography. J Chromatogr A 1371:257–260. https://doi.org/10.1016/j.chroma.2014.10.073
Acknowledgements
We acknowledge financial support from the projects of the National Natural Science Foundation of China (22004109, 22276177, and 21974124) and the China Postdoctoral Science Foundation (2022M710167).
Author information
Authors and Affiliations
Contributions
Wenfen Zhang: conceptualization, methodology, supervision, writing—original draft, funding acquisition, and project administration. Yumin Feng: validation and investigation. Long Pan: methodology and experiment supplement. Guangrui Zhang: methodology and experiment supplement. Yanhao Zhang: experiment supplement. Wuduo Zhao: experiment supplement. Zhengkun Xie: visualization. Shusheng Zhang: project administration, writing—review and editing, supervision, and funding acquisition
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, W., Feng, Y., Pan, L. et al. Silica microparticles modified with ionic liquid bonded chitosan as hydrophilic moieties for preparation of high-performance liquid chromatographic stationary phases. Microchim Acta 190, 176 (2023). https://doi.org/10.1007/s00604-023-05755-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-023-05755-6