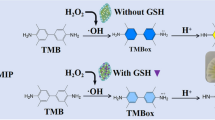
Abstract
An elaborate composite of molecularly imprinted polymer (MIP)-modified gold nanoparticles (AuNPs)@silica dioxide (SiO2) was designed and prepared for real-time colorimetric determination of glutathione (GSH) in serum. Firstly, the MIPs were synthesized on the surface of SiO2 utilizing GSH as template molecules. Then, AuNPs were synthesized on the surface of MIPs@SiO2 to produce a composite of MIPs modified by AuNPs@SiO2. Compared with plain AuNPs, the composite possessed better peroxidase catalysis activity due to stabilization and protection from hydrophilic SiO2, which can catalyze H2O2 to·OH oxidizing 3,3,5,5-tetramethylbenzidine (TMB) to the colored product. In addition, its selectivity was enhanced by MIP modification with special recognition cavities. With the composite as the sensor, GSH was precisely and sensitively detected in the range 5 ~ 40 μM with a limit of determination of 1.16 μM according to the principle of inhibitive peroxidase catalysis activity by GSH. The proposed colorimetric detection was successfully utilized for selective, convenient, and rapid determination of GSH in serum. It provided a new strategy for drug real-time monitoring and has high potential in clinical drug analysis.
Graphical Abstract






Similar content being viewed by others
References
Luo Y, Zhao X, Cai P et al (2020) One-pot synthesis of an anionic cyclodextrin-stabilized bifunctional gold nanoparticles for visual chiral sensing and catalytic reduction. Carbohydr Polym 237:116127. https://doi.org/10.1016/j.carbpol.2020.116127
Feng M, Zhang Q, Chen X et al (2022) Controllable synthesis of boron-doped Zn-N-C single-atom nanozymes for the ultrasensitive colorimetric detection of p-phenylenediamine. Biosens Bioelectron 210:114294. https://doi.org/10.1016/j.bios.2022.114294
D Zhang, P Du, J Chen et al (2021) Pyrazolate-based porphyrinic metal-organic frameworks as catechol oxidase mimic enzyme for fluorescent and colorimetric dual-mode detection of dopamine with high sensitivity and specificity. Sensors Actuators B Chem 341. https://doi.org/10.1016/j.snb.2021.130000
Wang M, Zhou X, Wang S et al (2021) Fabrication of bioresource-derived porous carbon-supported iron as an efficient oxidase mimic for dual-channel biosensing. Anal Chem 93:3130–3137. https://doi.org/10.1021/acs.analchem.0c04386
Liu X, Wei W, Yuan Q et al (2012) Apoferritin-CeO2 nano-truffle that has excellent artificial redox enzyme activity. Chem Commun (Camb) 48:3155–3157. https://doi.org/10.1039/c1cc15815e
Tu C, Lu H, Zhou T et al (2022) Promoting the healing of infected diabetic wound by an anti-bacterial and nano-enzyme-containing hydrogel with inflammation-suppressing. ROS-scavenging, Oxygen Nitric Oxide-generating Prop, Biomater 286:121597. https://doi.org/10.1016/j.biomaterials.2022.121597
Chen Y, Cai Y, Yu X et al (2021) A photo and tumor microenvironment activated nano-enzyme with enhanced ROS generation and hypoxia relief for efficient cancer therapy. J Mater Chem B 9:8253–8262. https://doi.org/10.1039/d1tb01437d
Ding H, Hu B, Zhang B et al (2020) Carbon-based nanozymes for biomedical applications. Nano Res 14:570–583. https://doi.org/10.1007/s12274-020-3053-9
Drozd M, Pietrzak M, Parzuchowski PG et al (2016) Pitfalls and capabilities of various hydrogen donors in evaluation of peroxidase-like activity of gold nanoparticles. Anal Bioanal Chem 408:8505–8513. https://doi.org/10.1007/s00216-016-9976-z
Ahmed HB (2019) Recruitment of various biological macromolecules in fabrication of gold nanoparticles: overview for preparation and applications. Int J Biol Macromol 140:265–277. https://doi.org/10.1016/j.ijbiomac.2019.08.138
Li RS, Liu H, Chen BB et al (2016) Stable gold nanoparticles as a novel peroxidase mimic for colorimetric detection of cysteine. Anal Methods 8:2494–2501. https://doi.org/10.1039/c6ay00367b
Bing W, Sun H, Wang F et al (2018) Hydrogen-producing hyperthermophilic bacteria synthesized size-controllable fine gold nanoparticles with excellence for eradicating biofilm and antibacterial applications. J Mater Chem B 6:4602–4609. https://doi.org/10.1039/c8tb00549d
Vinita NR, Nirala R (2018) Prakash, One step synthesis of AuNPs@MoS2-QDs composite as a robust peroxidase — mimetic for instant unaided eye detection of glucose in serum, saliva and tear. Sens Actuators B Chem 263:109–119. https://doi.org/10.1016/j.snb.2018.02.085
Zhang S, Gai S, He F et al (2014) Uniform Ni/SiO2@Au magnetic hollow microspheres: rational design and excellent catalytic performance in 4-nitrophenol reduction. Nanoscale 6:7025–7032. https://doi.org/10.1039/c4nr00338a
Chen L, Wang X, Lu W et al (2016) Molecular imprinting: perspectives and applications. Chem Soc Rev 45:2137–2211. https://doi.org/10.1039/c6cs00061d
Z Song, J Li, W Lu et al (2022) Molecularly imprinted polymers based materials and their applications in chromatographic and electrophoretic separations, TrAC Trends in Anal Chem 146. https://doi.org/10.1016/j.trac.2021.116504
Meseguer-Lloret S, Torres-Cartas S, Gomez-Benito C et al (2022) Magnetic molecularly imprinted polymer for the simultaneous selective extraction of phenoxy acid herbicides from environmental water samples. Talanta 239:123082. https://doi.org/10.1016/j.talanta.2021.123082
Song Z, Zhai X, Jiang C et al (2022) Sensitive and selective detection of carbamazepine in serum samples by bionic double-antibody sandwich method based on cucurbit[7]uril and molecular imprinted polymers. Biosens Bioelectron 203:114037. https://doi.org/10.1016/j.bios.2022.114037
Shahar T, Sicron T, Mandler D (2017) Nanosphere molecularly imprinted polymers doped with gold nanoparticles for high selectivity molecular sensors. Nano Res 10:1056–1063. https://doi.org/10.1007/s12274-016-1366-5
Zhang Z, Zhang X, Liu B et al (2017) Molecular imprinting on inorganic nanozymes for hundred-fold enzyme specificity. J Am Chem Soc 139:5412–5419. https://doi.org/10.1021/jacs.7b00601
Zhou J, Sheth S, Zhou H et al (2020) Highly selective detection of L-phenylalanine by molecularly imprinted polymers coated Au nanoparticles via surface-enhanced Raman scattering. Talanta 211:120745. https://doi.org/10.1016/j.talanta.2020.120745
Guo L, Zheng H, Zhang C et al (2020) A novel molecularly imprinted sensor based on PtCu bimetallic nanoparticle deposited on PSS functionalized graphene with peroxidase-like activity for selective determination of puerarin. Talanta 210:120621. https://doi.org/10.1016/j.talanta.2019.120621
Li L, Shi L, Jia J et al (2020) Dual photoluminescence emission carbon dots for ratiometric fluorescent GSH sensing and cancer cell recognition. ACS Appl Mater Interfaces 12:18250–18257. https://doi.org/10.1021/acsami.0c00283
Lopez-Navarro ME, Jarquin-Martinez M, Sanchez-Labastida LA et al (2020) Decoding aging: understanding the complex relationship among aging, free radicals, and GSH. Oxid Med Cell Longev 2020:3970860. https://doi.org/10.1155/2020/3970860
Curcio MF, Batista WL, Castro ED et al (2019) Nitric oxide stimulates a PKC-Src-Akt signaling axis which increases human immunodeficiency virus type 1 replication in human T lymphocytes. Nitric Oxide 93:78–89. https://doi.org/10.1016/j.niox.2019.09.004
F Nuhu, A Gordon, R Sturmey et al (2020) Measurement of glutathione as a tool for oxidative stress studies by high performance liquid chromatography, Molecules 25. https://doi.org/10.3390/molecules25184196
Zhang J, Yang H, Pan S et al (2021) A novel “off-on-off” fluorescent-nanoprobe based on B, N co-doped carbon dots and MnO2 nanosheets for sensitive detection of GSH and Ag+. Spectrochim Acta A Mol Biomol Spectrosc 244:118831. https://doi.org/10.1016/j.saa.2020.118831
Y Zheng, D Xu, L Sun et al (2022) Construction of a bioinspired Fe3O4/N-HCS nanozyme for highly sensitive detection of GSH, Colloids and Surfaces A: Physicochemical and Engineering Aspects 648. https://doi.org/10.1016/j.colsurfa.2022.129046
J Li, L Jiao, W Xu et al (2021) Cobalt oxyhydroxide nanosheets integrating with metal indicator enable sensitive detection of glutathione. Sensors Actuators B Chem 329. https://doi.org/10.1016/j.snb.2020.129247
J Xu, D Ren, N Chen et al (2021) A facile cooling strategy for the preparation of silica nanoparticles with rough surface utilizing a modified Stöber system, Colloids and Surfaces A: Physicochemical and Engineering Aspects 625. https://doi.org/10.1016/j.colsurfa.2021.126845
Tao Y, Ju E, Ren J et al (2015) Bifunctionalized mesoporous silica-supported gold nanoparticles: intrinsic oxidase and peroxidase catalytic activities for antibacterial applications. Adv Mater 27:1097–1104. https://doi.org/10.1002/adma.201405105
Liu J, Meng L, Fei Z et al (2017) MnO2 nanosheets as an artificial enzyme to mimic oxidase for rapid and sensitive detection of glutathione. Biosens Bioelectron 90:69–74. https://doi.org/10.1016/j.bios.2016.11.046
D’souza SL, Pati R, Kailasa SK (2014) Ascorbic acid-functionalized Ag NPs as a probe for colorimetric sensing of glutathione, Applied. Nanoscience 5:747–753. https://doi.org/10.1007/s13204-014-0371-9
Liu L, Jiang H, Wang X (2019) Bivalent metal ions tethered fluorescent gold nanoparticles as a reusable peroxidase mimic nanozyme. J Anal Test 3:269–276. https://doi.org/10.1007/s41664-019-00109-9
Y Wang, X Liu, M Wang et al (221) Facile synthesis of CDs@ZIF-8 nanocomposites as excellent peroxidase mimics for colorimetric detection of H2O2 and glutathione. Sensors Actuators B Chem 329. https://doi.org/10.1016/j.snb.2020.129115
W Yang, C Weng, X Li et al (2021) A sensitive colorimetric sensor based on one-pot preparation of h-Fe3O4@ppy with high peroxidase-like activity for determination of glutathione and H2O2. Sensors Actuators B Chem 338. https://doi.org/10.1016/j.snb.2021.129844
Xian Z, Zhang L, Yu Y et al (2021) Nanozyme based on CoFe2O4 modified with MoS2 for colorimetric determination of cysteine and glutathione. Mikrochim Acta 188:65. https://doi.org/10.1007/s00604-021-04702-7
S Wang, M Wang, Y Liu et al (2021) Novel D-π-A conjugated microporous polymer as visible light-driven oxidase mimic for efficient colorimetric detection of glutathione. Sensors Actuators B Chem 326. https://doi.org/10.1016/j.snb.2020.128808
Funding
This research was financially supported by the Doctoral Scientific Research Foundation of Jining Medical University (No. 6001/600761003), Research Fund for Lin He’s Academician Workstation of New Medicine and Clinical Translation in Jining Medical University (JYHL2018MS16), Supporting Fund for Teachers’ Research of Jining Medical University (JYFC2019KJ029), the National Innovation and Entrepreneurship Training Program for Undergraduate (No. 202210316026Z), and the Innovation and Entrepreneurship Training Program for Undergraduate (No. 202210316316).
Author information
Authors and Affiliations
Contributions
Conceptualization: Xiaoni Zhang, methodology: Jun Peng, formal analysis and investigation: Liping Xi, writing—original draft preparation: Ziwei Lu, writing—review and editing: LiLi Yu, funding acquisition: Meiru Liu, resources: Dezhi Huo, and supervision: Hua He.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, X., Peng, J., Xi, L. et al. Molecularly imprinted polymers enhanced peroxidase-like activity of AuNPs for determination of glutathione. Microchim Acta 189, 457 (2022). https://doi.org/10.1007/s00604-022-05576-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-022-05576-z