Abstract
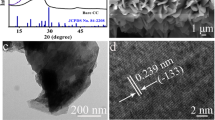
Cobalt hydroxide nanoparticles (Co(OH)2 NPs) were uniformly deposited on flexible carbon cloth substrate (Co(OH)2@CC) rapidly by a facile one-step electrodeposition, which can act as an enzyme-free glucose and uric acid sensor in an alkaline electrolyte. Compositional and morphological characterization were examined by X-ray diffraction (XRD), scanning electron microscopy (SEM), and energy-dispersive X-ray spectroscopy (EDS), which confirmed the deposited nanospheres were Co(OH)2 nanoparticles (NPs). The electrochemical oxidation of glucose and uric acid at Co(OH)2@CC electrode was investigated by electrochemical impedance spectroscopy (EIS), cyclic voltammetry (CV), differential pulse voltammetry (DPV), and chronoamperometry methods. The results revealed a remarkable electrocatalytic activity toward the single and simultaneous determination of glucose and uric acid at about 0.6 V and 0.3 V (vs. Ag/AgCl), respectively, which is attributed to a noticeable synergy effect between Co(OH)2 NPs and CC with good repeatability, satisfactory reproducibility, considerable long-term stability, superior selectivity, outstanding sensitivity, and wide linear detection range from 1 uM to 2 mM and 25 nM to 1.5 uM for glucose and UA, respectively. The detection limits were 0.36 nM for UA and 0.24 μM for glucose (S/N = 3). Finally, the Co(OH)2@CC electrode was utilized for glucose and uric acid determination in human blood samples and satisfying results were obtained. The relative standard derivations (RSDs) for glucose and UA were in the range 6 to 14% and 0 to 3%, respectively. The recovery ranges for glucose an UA were 97 to 103% and 95 and 101%, respectively. These features make the novel Co(OH)2@CC sensor developed by a low-cost, efficient, and eco-friendly preparation method a potentially practical candidate for application to biosensors.
Graphical abstract







Similar content being viewed by others
References
Zhao MG, Shang JH, Qu HY, Gao RJ, Li H, Chen SG (2020) Fabrication of the Ni/ZnO/BiOI foam for the improved electrochemical biosensing performance to glucose. Anal Chim Acta 1095:93–98. https://doi.org/10.1016/j.aca.2019.10.033
Liu SL, Zeng W, Li YQ (2020) Synthesis of ZnCo2O4 microrods grown on nickel foam for non-enzymatic glucose sensing. Mater Lett 259:126820. https://doi.org/10.1016/j.matlet.2019.126820
Hazhir T, Abbas B, Joseph W (2020) Electrochemical glucose sensors in diabetes management: an updated review (2010–2020). Chem Soc Rev 49(21):7671–7709. https://doi.org/10.1039/D0CS00304B
Vasiliou F, Plessas AK, Economou A, Thomaidis N, Papaefstathiou GS, Kokkinos C (2022) Graphite paste sensor modified with a Cu(II)-complex for the enzyme-free simultaneous voltammetric determination of glucose and uric acid in sweat. J Electroanal Chem 917:116393. https://doi.org/10.1016/j.jelechem.2022.116393
Zhou ZP, Shu T, Sun YF, Si HX, Peng PW, Su L, Zhang XJ (2021) Luminescent wearable biosensors based on gold nanocluster networks for “turn-on” detection of uric acid, glucose and alcohol in sweat. Biosens Bioelectron 192:113530. https://doi.org/10.1016/j.bios.2021.113530
Ahmad R, Tripathy N, Ahn MS, Hahn YB (2017) Solution process synthesis of high aspect ratio ZnO nanorods on electrode surface for sensitive electrochemical detection of uric acid. Sci Rep 7:46475. https://doi.org/10.1038/srep46475
Huang S, Yang EL, Yao JD, Chu X, Liu Y, Zhang Y, Xiao Q (2019) Nitrogen, cobalt Co-doped fluorescent magnetic carbon dots as ratiometric fluorescent probes for cholesterol and uric acid in human blood serum. ACS Omega 4(5):9333–9342. https://doi.org/10.1021/acsomega.9b00874
Balasubramanian P, Annalakshmi M, Chen SM, Chen TW (2019) Ultrasensitive non-enzymatic electrochemical sensing of glucose in noninvasive samples using interconnected nanosheets-like NiMnO3 as a promising electrocatalyst. Sens Actuators B Chem 299:126974. https://doi.org/10.1016/j.snb.2019.126974
Asif M, Aziz A, Ashraf G, Wang ZY, Wang JL, Azeem M, Chen XD, Xiao F, Liu HF (2018) Facet-inspired core-shell gold nanoislands on metal oxide octadecahedral heterostructures: high sensing performance toward sulfide in biotic fluids. ACS Appl Mater Interfaces 10(43):36675–36685. https://doi.org/10.1021/acsami.8b12186
Asif M, Wang HT, Dong S, Aziz A, Zhang GA, Xiao F, Liu HF (2017) Metal oxide intercalated layered double hydroxide nanosphere: with enhanced electrocatalyic activity towards H2O2 for biological applications. Sens Actuators B Chem 239:243–252. https://doi.org/10.1016/j.snb.2016.08.010
Aziz A, Asif M, Azeem M, Ashraf G, Wang ZY, Xiao F, Liu HF (2019) Self-stacking of exfoliated charged nanosheets of LDHs and graphene as biosensor with real-time tracking of dopamine from live cells. Anal Chim Acta 1047:197–207. https://doi.org/10.1016/j.aca.2018.10.008
Asif M, Aziz A, Wang HT, Wang ZY, Wang W, Ajmal M, Xiao F, Chen XD, Liu HF (2019) Superlattice stacking by hybridizing layered double hydroxide nanosheets with layers of reduced graphene oxide for electrochemical simultaneous determination of dopamine, uric acid and ascorbic acid. Mikrochim Acta 186(2):61. https://doi.org/10.1007/s00604-018-3158-y
Wu MY, Zhu JW, Ren YF, Yang N, Hong Y, Wang WJ, Huang W, Si WL, Dong XC (2019) NH2-GQDs-doped nickel-cobalt oxide deposited on carbon cloth for nonenzymatic detection of glucose. Adv Mater Interfaces 7(1):1901578. https://doi.org/10.1002/admi.201901578
Gupta J, Arya S, Verma S, Singh A, Sharma A, Singh B, Prerna Sharma R (2019) Performance of template-assisted electrodeposited Copper/Cobalt bilayered nanowires as an efficient glucose and uric acid senor. Mater Chem Phys 238:121969. https://doi.org/10.1016/j.matchemphys.2019.121969
Asif M, Aziz A, Wang ZY, Ashraf G, Wang JL, Luo HB, Chen XD, Xiao F, Liu HF (2019) Hierarchical CNTs@CuMn layered double hydroxide nanohybrid with enhanced electrochemical performance in H2S detection from live cells. Anal Chem 91(6):3912–3920. https://doi.org/10.1021/acs.analchem.8b04685
Aziz A, Asif M, Ashraf G, Iftikhar T, Hu JL, Xiao F, Wang SQ (2022) Boosting electrocatalytic activity of carbon fiber@fusiform-like copper-nickel LDHs: sensing of nitrate as biomarker for NOB detection. J Hazard Mater 422:126907. https://doi.org/10.1016/j.jhazmat.2021.126907
Javad T, Sayedeh Fatemeh NA, Mojtaba S (2018) A new bifunctional nanostructure based on two-dimensional nanolayered of Co(OH)2 exfoliated graphitic carbon nitride as a high performance enzyme-less glucose sensor: impedimetric and amperometric detection. Anal Chim Acta 1034:63–73. https://doi.org/10.1016/j.aca.2018.06.052
Wang Q, Chen YL, Zhu RX, Luo MF, Zou ZR, Yu HM, Jiang X, Xiong XL (2020) One-step synthesis of Co(OH)F nanoflower based on micro-plasma: as an effective non-enzymatic glucose sensor. Sens Actuators B Chem 304:127282. https://doi.org/10.1016/j.snb.2019.127282
Liu TT, Li M, Dong P, Zhang YJ, Guo LP (2018) Design and facile synthesis of mesoporous cobalt nitride nanosheets modified by pyrolytic carbon for the nonenzymatic glucose detection. Sens Actuators B Chem 255:1983–1994. https://doi.org/10.1016/j.snb.2017.08.218
Iman S, Umakant P, Atiye P, Nanasaheb MS, Seongil I, Seong CJ (2016) Enhanced non-enzymatic amperometric sensing of glucose using Co(OH)2 nanorods deposited on a three dimensional graphene network as an electrode material. Microchim Acta 183(8):2473–2479. https://doi.org/10.1007/s00604-016-1890-8
Asif M, Aziz A, Ashraf G, Iftikhar T, Sun YM, Xiao F, Liu HF (2022) Unveiling microbiologically influenced corrosion engineering to transfigure damages into benefits: a textile sensor for H2O2 detection in clinical cancer tissues. Chem Eng J 427:131398. https://doi.org/10.1016/j.cej.2021.131398
Litkohi HR, Bahari A, Ojani R (2017) Synthesis of Pt-Ni-Fe/CNT/CP nanocomposite as an electrocatalytic electrode for PEM fuel cell cathode. J Nanoparticle Res 19(8):278. https://doi.org/10.1007/s11051-017-3969-5
Zhang LJ, Wang N, Cao PF, Lin M, Xu L, Ma HY (2020) Electrochemical non-enzymatic glucose sensor using ionic liquid incorporated cobalt-based metal-organic framework. Microchem J 159:105343. https://doi.org/10.1016/j.microc.2020.105343
Pak M, Moshaii A, Siampour H, Abbasian S, Nikkhah M (2020) Cobalt-copper bimetallic nanostructures prepared by glancing angle deposition for non-enzymatic voltammetric determination of glucose. Mikrochim Acta 187(5):276. https://doi.org/10.1007/s00604-020-04246-2
Rios-Reyes CH, Granados-Neri M, Mendoza-Huizar LH (2009) Kinetic study of the cobalt electrodeposition onto glassy carbon electrode from ammonium sulfate solutions. Quim Nova 32(9):2382–2386. https://doi.org/10.1590/s0100-40422009000900028
Hu FX, Hu T, Chen SL, Wang DP, Rao QH, Liu YH, Dai FY, Guo CX, Yang HB, Li CM (2020) Single-atom cobalt-based electrochemical biomimetic uric acid sensor with wide linear range and ultralow detection limit. Nanomicro Lett 13(1):7. https://doi.org/10.1007/s40820-020-00536-9
Choi T, Kim SH, Lee CW, Kim H, Choi SK, Kim SH, Kim E, Park J, Kim H (2015) Synthesis of carbon nanotube-nickel nanocomposites using atomic layer deposition for high-performance non-enzymatic glucose sensing. Biosens Bioelectron 63:325–330. https://doi.org/10.1016/j.bios.2014.07.059
Asif M, Liu HW, Aziz A, Wang HT, Wang ZY, Ajmal M, Xiao F, Liu HF (2017) Core-shell iron oxide-layered double hydroxide: high electrochemical sensing performance of H2O2 biomarker in live cancer cells with plasma therapeutics. Biosens Bioelectron 97:352–359. https://doi.org/10.1016/j.bios.2017.05.057
Duan XX, Liu KL, Xu Y, Yuan MT, Gao T, Wang J (2019) Nonenzymatic electrochemical glucose biosensor constructed by NiCo2O4@Ppy nanowires on nickel foam substrate. Sens Actuators B Chem 292:121–128. https://doi.org/10.1016/j.snb.2019.04.107
Hoan NTV, Minh NN, Trang NTH, Thuy LT, Van Hoang C, Mau TX, Vu HXA, Thu PTK, Phong NH, Khieu DQ (2020) Simultaneous voltammetric determination of uric acid, xanthine, and hypoxanthine using CoFe2O4/reduced graphene oxide-modified electrode. J Nanomater 2020:1–15. https://doi.org/10.1155/2020/9797509
Abu Zahed M, Barman SC, Toyabur RM, Sharifuzzaman M, Xuan X, Nah J, Park JY (2019) Ex situ hybridized hexagonal cobalt oxide nanosheets and RGO@MWCNT based nanocomposite for ultra-selective electrochemical detection of ascorbic acid, dopamine, and uric acid. J Electrochem Soc 166(6):304–311. https://doi.org/10.1149/2.0131906jes
Arsalan M, Awais A, Qiao XJ, Sheng QL, Zheng JB (2020) Preparation and comparison of colloid based Ni50Co50(OH)2/BOX electrocatalyst for catalysis and high performance nonenzymatic glucose sensor. Microchem J 159:105486. https://doi.org/10.1016/j.microc.2020.105486
Amin KM, Muench F, Kunz U, Ensinger W (2021) 3D NiCo-Layered double Hydroxide@Ni nanotube networks as integrated free-standing electrodes for nonenzymatic glucose sensing. J Colloid Interface Sci 591:384–395. https://doi.org/10.1016/j.jcis.2021.02.023
Setoudeh N, Jahani S, Kazemipour M, Foroughi MM, Nadiki HH (2020) Zeolitic imidazolate frameworks and cobalt-tannic acid nanocomposite modified carbon paste electrode for simultaneous determination of dopamine, uric acid, acetaminophen and tryptophan: investigation of kinetic parameters of surface electrode and its analytical performance. J Electroanal Chem 863:114045. https://doi.org/10.1016/j.jelechem.2020.114045
Manjula N, Vinothkumar V, Chen SM, Sangili A (2020) Simultaneous and sensitive detection of dopamine and uric acid based on cobalt oxide-decorated graphene oxide composite. J Mater Sci Mater Electron 31(15):12595–12607. https://doi.org/10.1007/s10854-020-03810-z
Mounesh MP, Kumara NYP, Jilani BS, Mruthyunjayachari CD, Reddy KRV (2019) Synthesis and characterization of tetra-ganciclovir cobalt (II) phthalocyanine for electroanalytical applications of AA/DA/UA. Heliyon 5(7):e01946. https://doi.org/10.1016/j.heliyon.2019.e01946
Xu YX, Gao TT, Liang YM, Xiao D (2020) Intercalation lithium cobalt oxide for the facile fabrication of a sensitive dopamine sensor. ChemElectroChem 7(5):1193–1200. https://doi.org/10.1002/celc.202000099
Liu LL, Liu L, Wang YL, Ye BC (2019) A novel electrochemical sensor based on bimetallic metal-organic framework-derived porous carbon for detection of uric acid. Talanta 199:478–484. https://doi.org/10.1016/j.talanta.2019.03.008
Wang KD, Wu C, Wang F, Liao MH, Jiang GQ (2020) Bimetallic nanoparticles decorated hollow nanoporous carbon framework as nanozyme biosensor for highly sensitive electrochemical sensing of uric acid. Biosens Bioelectron 150:111869. https://doi.org/10.1016/j.bios.2019.111869
Acknowledgements
We thank very much advanced analysis and testing center of Nanjing Forestry University for SEM measurements.
Funding
This work was financially supported by the Natural Science Foundation of the Jiangsu Higher Education Institutions of China (21KJA220004).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, F., Shi, F., Chen, C. et al. Electrochemical fabrication of Co(OH)2 nanoparticles decorated carbon cloth for non-enzymatic glucose and uric acid detection. Microchim Acta 189, 385 (2022). https://doi.org/10.1007/s00604-022-05437-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-022-05437-9