Abstract
Thyroid-stimulating hormone (TSH) plays a crucial physiological and pathological role in humans, and a timely and sensitive detection of TSH is critical for early diagnosis and prevention of thyroid-related diseases. Herein, we developed a simple wash-free biological aptasensor based on luminescence resonance energy transfer (LRET) between NaYF4:Yb,Er upconversion nanoparticles (UCNPs) and tetramethylrhodamine (TAMRA) for the detection of TSH with high sensitivity. In this LRET system, UCNPs as donors and TAMRA as receptors were modified with nucleic acid aptamers Apt-1 and Apt-2, respectively. When TSH was present, the two aptamer strands both specifically recognized TSH to form a hairpin-like structure, thereby shortening the space between UCNPs and TAMRA. The LRET occurred under radiation of 980-nm light. By detecting the change of upconversion luminescence (UCL) intensity (I545nm), the activity of TSH was quantified. The resulting detection dynamic range and the limit of detection were 0.1–5.0 mIU·L−1 and 0.065 mIU·L−1, respectively. The aptasensor using UCNPs as LRET donors was capable of effectively eliminating the background interference of a complicated biological environment, and showed good specificity because of the excellent recognition function of aptamers. Due to high sensitivity, easiness of fabrication, operational convenience, and selectivity, the UCL-based aptasensor is a promising candidate for clinical TSH determination.
Graphical abstract
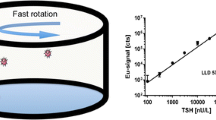
Based on nucleic acid aptamer and the mechanism of luminescence resonance energy transfer (LRET) between upconversion nanoparticles (UCNPs) donor and tetramethylrhodamine (TAMRA) receptor, an aptasensor was constructed for the quantitative analysis of TSH activity in serum by testing the change of I545nm.







Similar content being viewed by others
References
Smaniotto A, Mezalira DZ, Zapp E et al (2017) Electrochemical immunosensor based on an azo compound for thyroid-stimulating hormone detection. Microchem J 133:510–517
Liu Y, Zhang Q, Wang H et al (2015) An electrochemiluminescence immunosensor for thyroid stimulating hormone based on polyamidoamine-norfloxacin functionalized Pd-Au core-shell hexoctahedrons as signal enhancers. Biosens Bioelectron 71:164–170
Ross DS (1994) Hyperthyroidism, thyroid hormone therapy, and bone. Thyroid 4(3):319–326
Leung AM (2015) Subclinical hyperthyroidism is associated with increased risks of hip fractures, fractures at any site, nonspine fractures, and clinical spine fractures in the largest meta-analysis to date. Clinical Thyroidology 27(7):174–176
Kochupillai N, Mehta M (2008) Iodine deficiency disorders and their prevention in India. Rev Endocr Metab Disord 9(3):237
De Leo S, Lee SY, Braverman LE (2016) Hyperthyroidism. The Lancet 388:906–918
Kumar K, Mishra SK, Dwivedi P et al (2019) Recent progress in the sensing techniques for the detection of human thyroid stimulating hormone. Anal Chem 118:666–676
Choi S, Hwang J, Lee S et al (2017) Quantitative analysis of thyroid-stimulating hormone (TSH) using SERS-based lateral flow immunoassay. Sens Actuators, B Chem 240:358–364
Galofre JC, Duntas LH, Premawardhana LD et al (2012) Advances in Graves’ disease. J Thyroid Res 2012:809231
Wang R, Jia ZP, Hu XL et al (2003) Determination of serum thyroxine enantiomers in patients by liquid chromatography with a chiral mobile phase. J Chromatogr B Analyt Technol Biomed Life Sci 785(2):353–359
Tieu MV, Go A, Park YJ et al (2019) Highly sensitive ELISA using membrane-based microwave-mediated electrochemical immunoassay for thyroid-stimulating hormone detection. Ieee Sens J 19(21):9826–9831
Kosack CS, Page AL, Van Hulsteijn LT et al (2012) TSH-CHECK-1 test: diagnostic accuracy and potential application to initiating treatment for hypothyroidism in patients on anti-tuberculosis drugs. Plos one 7(3):e33704
Demers LM, Spencer CAJCE (2002) Laboratory support for the diagnosis and monitoring of thyroid disease. Clinical Endocrinology 58(2):138–140
Beitollahi H, Ivari SG, Torkzadeh-Mahani M (2018) Application of antibody-nanogold-ionic liquid-carbon paste electrode for sensitive electrochemical immunoassay of thyroid-stimulating hormone. Biosens Bioelectron 110:97–102
Ala-Kleme, Luminescence TJJo (2017) Heterogeneous time-resolved electrochemiluminoimmunoassay of thyroid stimulating hormone with magnetic beads at oxide-covered aluminum electrode. J Lumin 186:183–188
Nayl AA, Ibrahim AIAEA, El-Moghazy AY et al (2020) The nanomaterials and recent progress in biosensing systems: a review. Trends Environ Anal 26:87
Li CY, Zheng B, Li JT et al (2021) Holographic optical tweezers and boosting upconversion luminescent resonance energy transfer combined clustered regularly interspaced short palindromic repeats (CRISPR)/Cas12a biosensors. ACS Nano 15(5):8142–8154
Li Y, Chen C, Liu F et al (2022) Engineered lanthanide-doped upconversion nanoparticles for biosensing and bioimaging application. Mikrochim Acta 189(3):109
Zhu XJ, Su QQ, Feng W et al (2017) Anti-Stokes shift luminescent materials for bio-applications. Chem Soc Rev 46(4):1025–1039
Wu S, Butt HJ (2016) Near-infrared-sensitive materials based on upconverting nanoparticles. Adv Mater 28(6):1208–1226
Zhou B, Shi BY, Jin DY et al (2015) Controlling upconversion nanocrystals for emerging applications. Nat Nanotechnol 10(11):924–936
Christie RJ, Tadiello CJ, Chamberlain LM et al (2009) Optical properties and application of a reactive and bioreducible thiol-containing tetramethylrhodamine dimer. Bioconjug Chem 20(3):476–480
Kusamoto H, Shiba A, Tsunehiro M et al (2018) A simple method for determining the ligand affinity toward a zinc-enzyme model by using a TAMRA/TAMRA interaction. Dalton Trans 47(6):1841–1848
Han S, Deng R, Xie X et al (2014) Enhancing luminescence in lanthanide-doped upconversion nanoparticles. Angew Chem Int Ed 53(44):11702–11715
Nimjee SM, White RR, Becker RC et al (2017) Aptamers as therapeutics. Annu Rev Pharmacol Toxicol 57:61–79
Yuan Y, Gu Z, Yao C et al (2019) Nucleic acid-based functional nanomaterials as advanced cancer therapeutics [J]. Small 15(26):e1900172
Zhou Q, Rahimian A, Son K et al (2016) Development of an aptasensor for electrochemical detection of exosomes. Methods 97:88–93
Macdonald J, Henri J, Goodman L et al (2017) Development of a bifunctional aptamer targeting the transferrin receptor and epithelial cell adhesion molecule (EpCAM) for the treatment of brain cancer metastases. ACS Chem Neurosci 8(4):777–784
Zhu H, Ding YJ, Wang AQ et al (2014) A simple strategy based on upconversion nanoparticles for fluorescent resonant energy transfer biosensor. J Mater Chem B 3(3):458–464
Dong AG, Ye XC, Chen J et al (2011) A generalized ligand-exchange strategy enabling sequential surface functionalization of colloidal nanocrystals. J Am Chem Soc 133(4):998–1006
Muhr V, Würth C, Kraft M et al (2017) Particle-size-dependent Forster resonance energy transfer from upconversion nanoparticles to organic dyes. Anal Chem 89(9):4868–4874
Leipply D DE (2011) Draper, Evidence for a thermodynamically distinct Mg2+ ion associated with formation of an RNA tertiary structure. J Am Chem Soc 133(2011):13397
Tieu MV, Go A, Park YJ et al (2019) Highly sensitive ELISA using membrane-based microwave-mediated electrochemical immunoassay for thyroid-stimulating hormone detection. Ieee Sens J 19(21):9826–9831
Lin Z, Wang X, Li ZJ et al (2008) Development of a sensitive, rapid, biotin-streptavidin based chemiluminescent enzyme immunoassay for human thyroid stimulating hormone. Talanta 75(4):965–972
Asav E (2021) Development of a functional impedimetric immunosensor for accurate detection of thyroid-stimulating hormone. Turk J Chem 45(3):819–834
Jung W, Han J, Kai J et al (2013) An innovative sample-to-answer polymer lab-on-a-chip with on-chip reservoirs for the POCT of thyroid stimulating hormone (TSH). Lab Chip 13(23):4653–4662
Choi S, Hwang J, Lee S et al (2017) Quantitative analysis of thyroid-stimulating hormone (TSH) using SERS-based lateral flow immunoassay sensor. Actuat B-Chem 240:358–364
Trevino J, Calle A, Rodriguez-Frade JM et al (2009) Surface plasmon resonance immunoassay analysis of pituitary hormones in urine and serum samples Clin. Chim Acta 403(1–2):56–62
Lin ZH, Shen GL, Miao Q et al (1996) A thyroid-stimulating hormone immuno-electrode [J] 325(1–2):87–92
Lu N, Dai P, Gao A et al (2014) Label-free and rapid electrical detection of hTSH with CMOS-compatible silicon nanowire transistor arrays. ACS Appl Mater Interfaces 6(22):20378–20384
Wu FB, Han SQ, Xu T et al (2003) Sensitive time-resolved fluoroimmunoassay for simultaneous detection of serum thyroid-stimulating hormone and total thyroxin with Eu and Sm as labels. Anal Biochem 314(1):87–96
Rajesh KK, Mishra SK et al (2019) Recent progress in the sensing techniques for the detection of human thyroid stimulating hormone. Trends Anal Chem 118:666–676
Utiger RD (1965) Radioimmunoassay of human plasma thyrotropin. J Clin Investig 44(8):1277–1286
Funding
This research was supported in part by the National Natural Science Foundation of China (No. 21874019), Fujian Science and Technology Innovation Joint Found Project (No. 2019Y9008), Science and Technology Plan Guided Project of Fujian Provincial Science and Technology Department (No. 2020Y0022), and the Natural Science Foundation of Fujian Province (No. 2019J01534, 2020J01628).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, J., Yu, C., Han, L. et al. Upconversion luminescence–based aptasensor for the detection of thyroid-stimulating hormone in serum. Microchim Acta 189, 179 (2022). https://doi.org/10.1007/s00604-022-05279-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-022-05279-5