Abstract
A chemically stable Zr(IV)-based metal-organic framework (BUT-17) has been explored for simultaneous adsorption and determination of bisphenol compounds (BPs) in aqueous medium. The prepared BUT-17 possesses a large surface area (2936 m2 g−1) and excellent fluorescent performance. An adsorption capacity of 111 mg g−1 for bisphenol A (BPA) with a rapid adsorption rate (1.76 g mg−1 min−1) is achieved by BUT-17. The excellent adsorption performance could be attributed to the hydrogen bond interaction between BPs and BUT-17. Furthermore, the fluorescent intensity of BUT-17 was quenched up to 92% due to the formation of complexes between BPs and BUT-17. Thus, a BUT-17-based fluorescent sensing method for the rapid determination of BPs has been established with the limit of detection of 10.0 ng mL−1 for BPA and a linear range from 2.0 to 23.0 μg mL−1. These results indicate that as an outstanding multifunctional platform, BUT-17 is promising for the simultaneous removal and determination of BPs in water medium.
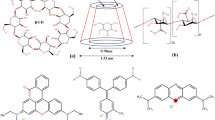
Graphical abstract

Simultaneous removal and detection of BPs with BUT-17.





Similar content being viewed by others
References
Street ME, Bernasconi S (2020) Endocrine-disrupting chemicals in human fetal growth. Int J Mol Sci 21(4):1430. https://doi.org/10.3390/ijms21041430
Marqueño A, Pérez-Albaladejo E, Flores C, Moyano E, Porte C (2019) Toxic effects of bisphenol a diglycidyl ether and derivatives in human placental cells. Environ Pollut 244:513–521. https://doi.org/10.1016/j.envpol.2018.10.045
Xue J, Liu W, Kannan K (2017) Bisphenols, benzophenones, and bisphenol a diglycidyl ethers in textiles and infant clothing. Environ Sci Technol 51:5279–5286. https://doi.org/10.1021/acs.est.7b00701
Liao C, Kannan K (2019) Species-specific accumulation and temporal trends of bisphenols and benzophenones in mollusks from the Chinese Bohai Sea during 2006–2015. Sci Total Environ 653:168–175. https://doi.org/10.1016/j.scitotenv.2018.10.271
Catron T, Keely S, Brinkman N, Zurlinden T, Wood C, Wright J, Phelps D, Wheaton E, Kvasnicka A, Gaballah S, Lamendella R, Tal T (2019) Host developmental toxicity of BPA and BPA alternatives is inversely related to microbiota disruption in zebrafish. Toxicol Sci 167:468–483. https://doi.org/10.1093/toxsci/kfy261
Gallartayala H, Moyano E, Galceran MT (2011) Analysis of bisphenols in soft drinks by on-line solid phase extraction fast liquid chromatography-tandem mass spectrometry. Anal Chim Acta 683:227–233. https://doi.org/10.1016/j.aca.2010.10.034
Chang W-H, Liu S-C, Chen H-L, Lee C-C (2019) Dietary intake of 4-nonylphenol and bisphenol a in Taiwanese population: integrated risk assessment based on probabilistic and sensitive approach. Environ Pollut 244:143–152. https://doi.org/10.1016/j.envpol.2018.10.040
Reyesgallardo E, Lucena R, Cardenas S, Valcarcel M (2016) Dispersive micro-solid phase extraction of bisphenol a from milk using magnetic nylon 6 composite and its final determination by HPLC-UV. Microchem J 124:751–756. https://doi.org/10.1016/j.microc.2015.10.025
Diao C, Yang X, Sun A, Liu R (2015) A combined technique for the pretreatment of ultra trace bisphenol a in environmental water based on magnetic matrix solid phase extraction assisted dispersive liquid-liquid microextraction. Anal Methods 7:10170–10176. https://doi.org/10.1039/C5AY02711J
Zhou XL, Kramer JP, Calafat AM, Ye XY (2014) Automated on-line column-switching high performance liquid chromatography isotope dilution tandem mass spectrometry method for the quantification of bisphenol a, bisphenol F, bisphenol S, and 11 other phenols in urine. J Chromatogr B 944:152–156. https://doi.org/10.1016/j.jchromb.2013.11.009
Liu Y, Wang D, Du F, Zheng W, Liu Z, Xu Z, Hu X, Liu H (2019) Dummy-template molecularly imprinted micro-solid-phase extraction coupled with high-performance liquid chromatography for bisphenol a determination in environmental water samples. Microchem J 145:337–344. https://doi.org/10.1016/j.microc.2018.10.054
Becerra V, Odermatt J (2012) Detection and quantification of traces of bisphenol a and bisphenol S in paper samples using analytical pyrolysis-GC/MS. Analysts 137:2250–2259. https://doi.org/10.1039/C2AN15961A
Sasaki N, Okuda K, Kato T, Kakishima H, Okuma H, Abe K, Tachino H, Tuchida K, Kubono K (2005) Salivary bisphenol-a levels detected by ELISA after restoration with composite resin. J Mater Sci Mater Med 16:297–300. https://doi.org/10.1007/s10856-005-0627-8
Diepens M, Gijsman P (2007) Photodegradation of bisphenol a polycarbonate. Polym Degrad Stab 92:397–406. https://doi.org/10.1016/j.polymdegradstab.2006.12.003
Xu J, Wang L, Zhu Y (2012) Decontamination of bisphenol a from aqueous solution by graphene adsorption. Langmuir 28:8418–8425. https://doi.org/10.1021/la301476p
Canevari T, Rossi M, Alexiou A (2019) Development of an electrochemical sensor of endocrine disruptor bisphenol a by reduced graphene oxide for incorporation of spherical carbon nanoparticles. J Electroanal Chem 832:24–30. https://doi.org/10.1016/j.jelechem.2018.10.044
Chen J, Huang X, Lee D (2008) Bisphenol a removal by a membrane bioreactor. Process Biochem 43:451–456. https://doi.org/10.1016/j.procbio.2008.01.001
Peng Y, Chen Y, Chang Y, Shi Y (2015) Biodegradation of bisphenol a with diverse microorganisms from river sediment. J Hazard Mater 286:285–290. https://doi.org/10.1016/j.jhazmat.2014.12.051
Wang P, Xie L, Joseph E, Li J, Su X, Zhou H (2019) Metal-organic frameworks for food safety. Chem Rev 119:10638–10690. https://doi.org/10.1021/acs.chemrev.9b00257
Bai Y, Dou Y, Xie L, Rutledge W, Li J, Zhou H (2016) Zr-based metal-organic frameworks: design, synthesis, structure, and applications. Chem Soc Rev 45:2327–2367. https://doi.org/10.1039/C5CS00837A
Qiao Y, Liu Q, Lu S, Chen G, Gao S, Lu W, Sun X (2020) High-performance non-enzymatic glucose detection: using a conductive Ni-MOF as an electrocatalyst. J Mater Chem B 8:5411–5415. https://doi.org/10.1039/D0TB00131G
Xiong W, Cheng X, Wang T, Luo Y, Feng J, Lu S, Asiri A, Li W, Jiang Z, Sun X (2020) Co3(hexahydroxytriphenylene)2: a conductive metal-organic framework for ambient electrocatalytic N2 reduction to NH3. Nano Res 13:1008–1012. https://doi.org/10.1007/s12274-020-2733-9
Park J, Jhung S (2020) A remarkable adsorbent for removal of bisphenol S from water: aminated metal-organic framework, MIL-101-NH2. Chem Eng J 396:125224. https://doi.org/10.1016/j.cej.2020.125224
Bůžek D, Ondrušová S, Hynek J, Kovář P, Lang K, Rohlíček J, Demel J (2020) Robust aluminum and iron phosphinate metal-organic frameworks for efficient removal of bisphenol a. Inorg Chem 59:5538–5545. https://doi.org/10.1021/acs.inorgchem.0c00201
Meng AN, Chaihu LX, Chen HH, Gu ZY (2017) Ultrahigh adsorption and singlet-oxygen mediated degradation for efficient synergetic removal of bisphenol a by a stable zirconium-porphyrin metal-organic framework. Sci Rep 7:6297. https://doi.org/10.1038/s41598-017-06194-z
Wang X, Lu X, Wu L, Chen J (2015) 3D metal-organic framework as highly efficient biosensing platform for ultrasensitive and rapid detection of bisphenol a. Biosens Bioelectron 65:295–301. https://doi.org/10.1016/j.bios.2014.10.010
Yang H, Wang B, Liu J, Cheng J, Yu L, Yu J, Wang P, Li J, Su X (2020) Sensitive and selective detection of bisphenol compounds in a fluorescent metal-organic framework. Sensors Actuators B Chem 314:128048. https://doi.org/10.1016/j.snb.2020.128048
Wang B, Wang P, Xie L, Lin R, Lv J, Li J, Chen B (2019) A stable zirconium-based metal-organic framework for specific recognition of representative polychlorinated dibenzo-p-dioxin molecules. Nat Commun 10:1–8. https://doi.org/10.1038/s41467-019-11912-4
Spek AL (2003) Single-crystal structure validation with the program PLATON. J Appl Crystallogr 36:7–13. https://doi.org/10.1107/S0021889802022112
Nagarkar S, Joarder B, Chaudhari A, Ghosh S, Mukherjee S (2013) Highly selective detection of nitro explosives by a luminescent metal-organic framework. Angew Chem Int Ed 52:2881–2885. https://doi.org/10.1002/anie.201208885
Alsbaiee A, Smith B, Xiao L, Ling Y, Helbling D, Dichtel W (2016) Rapid removal of organic micropollutants from water by a porous β-cyclodextrin polymer. Nature 529:190–194. https://doi.org/10.1038/nature16185
Ha N, Lefedova O, Ha N (2016) Theoretical study on the adsorption of carbon dioxide on individual and alkali-metal doped MOF-5s. Russ J Phys Chem A 90:220–225. https://doi.org/10.1134/S0036024415120201
Jin Z, Wang X, Sun Y, Ai Y, Wang X (2015) Adsorption of 4-n-nonylphenol and bisphenol-a on magnetic reduced graphene oxides: a combined experimental and theoretical studies. Environ Sci Technol 49:9168–9175. https://doi.org/10.1021/acs.est.5b02022
Abid HR, Ang HM, Wang SB (2012) Effects of ammonium hydroxide on the structure and gas adsorption of nanosized Zr-MOFs (UiO-66). Nanoscale 4:3089–3094. https://doi.org/10.1039/C2NR30244F
Dong X, Wang R, Wang J, Zang S, Mak T (2015) Highly selective Fe3+ sensing and proton conduction in a water-stable sulfonate-carboxylate Tb-organic-framework. J Mater Chem A 3:641–647. https://doi.org/10.1039/C4TA04421E
Xiang ZH, Fang CQ, Leng SH, Cao DP (2014) An amino group functionalized metal-organic framework as a luminescent probe for highly selective sensing of Fe3+ ions. J Mater Chem A 2:7662–7665. https://doi.org/10.1039/C4TA00313F
Acknowledgments
The authors would like to acknowledge the financial support from the National key research and development program of China (Grant No. 2017YFC1601604), the National Natural Science Foundation of China (Grant No. 21777189, 21576006, 21601008), the Science Fund for Creative Research Groups of the National Natural Science Foundation of China (Grant No. 51621003), the Beijing Natural Science Foundation (Grant No. 2182005), and Fundamental Research Funds for Central Public Research Institutes (Grant No. Y2020PT38).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 6837 kb)
Rights and permissions
About this article
Cite this article
Yu, L., Cheng, J., Yang, H. et al. Simultaneous adsorption and determination of bisphenol compounds in water medium with a Zr(IV)-based metal-organic framework. Microchim Acta 188, 83 (2021). https://doi.org/10.1007/s00604-021-04742-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-021-04742-z