Abstract
An ultrasensitive aptasensor is described for the voltammetric determination of the Mycobacterium tuberculosis antigen MPT64 in human serum. Firstly, an amino-modified Zr(IV) based metal-organic framework (MOF; type UiO-66-NH2; made up from Zr6O32 units and 2-amino-terephthalate linkers) with a high specific surface was synthesized and used as the carrier of the gold nanoparticles and the aptamers. Then the signalling nanoprobe was fabricated after the horseradish peroxidase was cast on the nanomaterials. The two aptamers with synergistic effect on binding MPT64 were anchored on the gold electrode. Differential pulse voltammetry indicated that the peak current is highest if the ratio of the two aptamers is 1:1. The assay has a wide linear response range (0.02 to 1000 pg·mL−1 of MPT64) and a 10 fg·mL−1 detection limit at a working potential of around −96 mV (vs Ag/AgCl). The results show this biosensor to be a viable tool for detection of tuberculosis at an early stage.

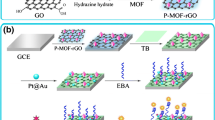
Schematic presentation of the construction of the nanoprobe and biosensor. Firstly, the surface of UiO-66-NH2 was anchored to gold nanoparticles (AuNPs). A dual-aptamer and HRP were added to form the signalling nanoprobe (Aptamer/HRP/AuNPs/UiO-66-NH2). Then, the aptamers I and II were attached on the surface of gold electrode and 6-mercapto-1-hexanol was used to block the uncovered active site of the gold electrode. Finally, after incubation with MPT64, the signalling nanoprobe was dropped on the modified electrode and the DPV measurements was used for the analysis of Mycobacterium tuberculosis antigen MPT64. (PVP: poly(vinyl pyrrolidone); HRP: horseradish peroxidase; MCH: 6-Mercapto-1-hexanol; HQ: hydroquinone; BQ: benzoquinone).




Similar content being viewed by others
References
Farid S, Meshik X, Choi M, Mukherjee S, Lan Y, Parikh D, Poduri S, Baterdene U, Huang CE, Wang YY, Burke P, Dutta M, Stroscio MA (2015) Detection of interferon gamma using graphene and aptamer based FET-like electrochemical biosensor. Biosens Bioelectron 71:294–299
Zumla A, George A, Sharma V, Herbert RHN, Masham of Ilton B, Oxley A, Oliver M (2015) The WHO 2014 global tuberculosis report-further to go. Lancet Glob Health 3:E10–E12
Mi XW, He FJ, Xiang MY, Lian Y, Yi SL (2012) Novel phage amplified multichannel series piezoelectric quartz crystal sensor for rapid and sensitive detection of Mycobacterium tuberculosis. Anal Chem 84:939–946
Mejia GI, Castrillon L, Trujillo H, Robledo JA (1999) Microcolony detection in 7H11 thin layer culture is an alternative for rapid diagnosis of Mycobacterium tuberculosis infection. Int J Tuberc Lung D 3:138–142
Moure R, Munoz L, Torres M, Santin M, Martin R, Alcaide F (2011) Rapid detection of Mycobacterium tuberculosis complex and rifampin resistance in smear-negative clinical samples by use of an integrated real-time PCR method. J Clin Microbiol 49:1137–1139
Ji M, Cho B, Cho YS, Park SY, Cho SN, Jeon BY, Yoon BS (2014) Development of a quantitative sandwich enzyme-linked immunosorbent assay for detecting the MPT64 antigen of Mycobacterium tuberculosis. Yonsei Med J 55(3):746–752
Mehaffy C, Dobos KM, Nahid P, Kruh-Garcia NA (2017) Second generation multiple reaction monitoring assays for enhanced detection of ultra-low abundance Mycobacterium tuberculosis peptides in human serum. Clin Proteomics 14:21
Theron G, Peter J, van Zyl-Smit R, Mishra H, Streicher E, Murray S, Dawson R, Whitelaw A, Hoelscher M, Sharma S, Pai M, Warren R, Dheda K (2011) Evaluation of the Xpert MTB/RIF assay for the diagnosis of pulmonary tuberculosis in a high HIV prevalence setting. Am J Respir Crit Care Med 184:132–140
Jiang Y, Liu HC, Wan KL (2014) MPT64 polymorphisms of Mycobacterium tuberculosis strains suggest ongoing immune evasion. Tuberculosis 94:712–714
Yin XM, Zheng L, Lin L, Hu YR, Zheng F, Hu YW, Wang Q (2013) Commercial MPT64-based tests for rapid identification of Mycobacterium tuberculosis complex: a meta-analysis. J Inf Secur 67:369–377
Thakur H, Kaur N, Sabherwal P, Sareen D, Prabhakar N (2017) Aptamer based voltammetric biosensor for the detection of Mycobacterium tuberculosis antigen MPT64. Microchim Acta 184(7):1915–1922
Gou D, Xie G, Li Y, Zhang X, Chen H (2018) Voltammetric immunoassay for Mycobacterium tuberculosis secretory protein MPT64 based on a synergistic amplification strategy using rolling circle amplification and a gold electrode modified with graphene oxide, Fe3O4 and Pt nanoparticles. Microchim Acta 185(9):1–9
Qin LH, Zheng RJ, Ma ZX, Feng YH, Liu ZH, Yang H, Wang J, Jin RL, Lu JM, Ding YS, Hu ZY (2009) The selection and application of ssDNA aptamers against MPT64 protein in Mycobacterium tuberculosis. Clin Chem Lab Med 47:405–411
Zhou M, Zhai YM, Dong SJ (2009) Electrochemical sensing and biosensing platform based on chemically reduced graphene oxide. Anal Chem 81:5603–5613
Yang J, Dou B, Yuan R, Xiang Y (2016) Proximity binding and metal ion-dependent DNAzyme cyclic amplification-integrated aptasensor for label-free and sensitive electrochemical detection of thrombin. Anal Chem 88:8218–8223
Sun DP, Lu J, Zhong YW, Yu YY, Wang Y, Zhang BB, Chen ZG (2016) Sensitive electrochemical aptamer cytosensor for highly specific detection of cancer cells based on the hybrid nanoelectrocatalysts and enzyme for signal amplification. Biosens Bioelectron 75:301–307
Zheng TT, Tan TT, Zhang QF, Fu JJ, Wu JJ, Zhang K, Zhu JJ, Wang H (2013) Multiplex acute leukemia cytosensing using multifunctional hybrid electrochemical nanoprobes at a hierarchically nanoarchitectured electrode interface. Nanoscale 5:10360–10368
Zheng TT, Zhang QF, Feng S, Zhu JJ, Wang Q, Wang H (2014) Robust nonenzymatic hybrid nanoelectrocatalysts for signal amplification toward ultrasensitive electrochemical cytosensing. J Am Chem Soc 136:2288–2291
Zhou HC, Long JR, Yaghi OM (2012) Introduction to metal-organic frameworks. Chem Rev 112:673–674
Gascon V, Castro-Miguel E, Diaz-Garcia M, Blanco RM, Sanchez-Sanchez M (2017) In situ and post-synthesis immobilization of enzymes on nanocrystalline MOF platforms to yield active biocatalysts. J Chem Technol Biotechnol 92:2583–2593
Ricco R, Pfeiffer C, Sumida K, Sumby CJ, Falcaro P, Furukawa S, Champness NR, Doonan CJ (2016) Emerging applications of metal-organic frameworks. Crystengcomm 18:6532–6542
Sabyrov K, Jiang J, Yaghi OM, Somorjai GA (2017) Hydroisonnerization of n-hexane using acidified metal-organic framework and platinum nanoparticles. J Am Chem Soc 139:12382–12385
Feng DW, Liu TF, Su J, Bosch M, Wei ZW, Wan W, Yuan DQ, Chen YP, Wang X, Wang KC, Lian XZ, Gu ZY, Park J, Zou XD, Zhou HC (2015) Stable metal-organic frameworks containing single-molecule traps for enzyme encapsulation. Nat Commun 6:5979
Li L, Yuan Y, Chen Y, Zhang P, Bai Y, Bai L (2018) Aptamer based voltammetric biosensor for Mycobacterium tuberculosis antigen ESAT-6 using a nanohybrid material composed of reduced graphene oxide and a metal-organic framework. Microchim Acta 185(8):379
Shen LJ, Wu WM, Liang RW, Lin R, Wu L (2013) Highly dispersed palladium nanoparticles anchored on UiO-66(NH2) metal-organic framework as a reusable and dual functional visible-light-driven photocatalyst. Nanoscale 5:9374–9382
Guo ZY, Xiao CX, Maligal-Ganesh RV, Zhou L, Goh TW, Li XL, Tesfagaber D, Thiel A, Huang WY (2014) Pt nanoclusters confined within metal organic framework cavities for chemoselective cinnamaldehyde hydrogenation. ACS Catal 4:1340–1348
Ling PH, Lei JP, Jia L, Ju HX (2016) Platinum nanoparticles encapsulated metal-organic frameworks for the electrochemical detection of telomerase activity. Chem Commun 52:1226–1229
Szymanski M, Noble J, Knight A, Porter R, Worsley G (2013) Aptamer-mediated detection of thrombin using silver nanoparticle signal enhancement. Anal Methods 5:187–191
Li XM, Liu JM, Zhang SS (2010) Electrochemical analysis of two analytes based on a dual-functional aptamer DNA sequence. Chem Commun 46:595–597
Min K, Song KM, Cho M, Chun YS, Shim YB, Ku JK, Ban C (2010) Simultaneous electrochemical detection of both PSMA (+) and PSMA (−) prostate cancer cells using an RNA/peptide dual-aptamer probe. Chem Commun 46:5566–5568
Qu LM, Xu JH, Tan XF, Liu Z, Xu LG, Peng R (2014) Dual-aptamer modification generates a unique interface for highly sensitive and specific electrochemical detection of tumor cells. ACS Appl Mater Interfaces 6:7309–7315
Gopinath SCB, Perumal V, Kumaresan R, Lakshmipriya T, Rajintraprasad H, Rao BS, Arshad MKM, Chen Y, Kotani N, Hashim U (2016) Nanogapped impedimetric immunosensor for the detection of 16 kDa heat shock protein against Mycobacterium tuberculosis. Microchim Acta 183(10):2697–2703
Bai L, Chen Y, Bai Y, Chen Y, Zhou J, Huang A (2017) Fullerenedoped polyaniline as new redox nanoprobe and catalyst in electrochemical aptasensor for ultrasensitive detection of Mycobacterium tuberculosis MPT64 antigen in human serum. Biomaterials 133:11–19
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 21675177), the Science and Technology Planning Project of Guangdong Province (No. 2016B030303002), the Natural Science Foundation of Guangdong Province (No. 2018A030310142), and the Medical Scientific Research Foundation of Guangdong Province (No. A2017033).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOC 565 kb)
Rights and permissions
About this article
Cite this article
Li, N., Huang, X., Sun, D. et al. Dual-aptamer-based voltammetric biosensor for the Mycobacterium tuberculosis antigen MPT64 by using a gold electrode modified with a peroxidase loaded composite consisting of gold nanoparticles and a Zr(IV)/terephthalate metal-organic framework. Microchim Acta 185, 543 (2018). https://doi.org/10.1007/s00604-018-3081-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-018-3081-2