Abstract
The authors describe an electrochemical immunoassay for ultrasensitive determination of the Mycobacterium tuberculosis (MTb) secretory protein MPT64 which is an antigen for early diagnosis of infection with MTb. Protein G was used to immobilize antibodies against MPT64 on a gold electrode. Graphene oxide with its large surface area was used as a carrier to anchor magnetite (Fe3O4) and platinum (Pt) nanoparticles. The nanocomposite of type GO@Fe3O4@Pt was used as a signal reporter with excellent catalytic activity and recyclability. The nanocomposite exhibits peroxidase-like activity for hydrogen peroxide, best at −0.25 V (vs. Ag/AgCl). It works over a wide range of pH values (2–10) and temperatures (25–65 °C). Further signal amplification strategy was accomplished by rolling circle amplification. This voltammetric assay has a linear response to the logarithm of MPT64 concentration in the range from 5.0 fg·mL−1 to 1.0 ng·mL−1 and a detection limit of 0.34 fg·mL−1 (at a signal to noise ratio of 3, for n = 10). The assay can be completed within 4 h. It was successfully applied to the determination of MPT64 in spiked serum samples. Conceivably, the assay has a large potential in providing laboratory evidence for rapid diagnosis of MTb infection.

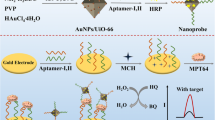
An electrochemical biosensor was developed for rapid detection of Mycobacterium tuberculosis secretory protein MPT64. The detection signal was synergistically amplified by GO@Fe3O4@Pt and rolling circle amplification. Phi29: phi29 DNA polymerase; BSA: bovine serum albumin; GO: graphene oxide.





Similar content being viewed by others
References
Turbawaty DK, Sugianli AK, Soeroto AY, Setiabudiawan B, Parwati I (2017) Comparison of the performance of urinary Mycobacterium tuberculosis antigens cocktail (ESAT6, CFP10, and MPT64) with culture and microscopy in pulmonary tuberculosis patients. Int J Microbiol 2017:1–5 https://doi.org/10.1155/2017/3259329
World Health Organization (2017) Global tuberculosis report 2017. Available from: http://wwwwhoint/tb/publications/global_report/en/ Accessed 10 March 2018
Caceres N, Vilaplana C, Prats C, Marzo E, Llopis I, Valls J, Lopez D, Cardona PJ (2013) Evolution and role of corded cell aggregation in Mycobacterium tuberculosis cultures. Tuberculosis (Edinb) 93(6):690–698 https://doi.org/10.1016/j.tube.2013.08.003
Steingart KR, Ng V, Henry M, Hopewell PC, Ramsay A, Cunningham J, Urbanczik R, Perkins MD, Aziz MA, Pai M (2006) Sputum processing methods to improve the sensitivity of smear microscopy for tuberculosis: a systematic review. Lancet Infect Dis 6(10):664–674 https://doi.org/10.1016/s1473-3099(06)70602-8
Wang X, Xie F, Zheng Q, Qi X, Li M, Zhou X, Zheng Z (2017) Fluorescent quantitative PCR detection of Mycobacterium tuberculosis in tissue sections from granulomatous lesions retrieved using EDTA. J Clin Pathol 70(5):390–394 https://doi.org/10.1136/jclinpath-2016-203738
Kim EJ, Kim EB, Lee SW, Cheon SA, Kim HJ, Lee J, Lee MK, Ko S, Park TJ (2017) An easy and sensitive sandwich assay for detection of Mycobacterium tuberculosis Ag85B antigen using quantum dots and gold nanorods. Biosens Bioelectron 87:150–156 https://doi.org/10.1016/j.bios.2016.08.034
Yin X, Zheng L, Lin L, Hu Y, Zheng F, Hu Y, Wang Q (2013) Commercial MPT64-based tests for rapid identification of Mycobacterium tuberculosis complex: a meta-analysis. J Inf Secur 67(5):369–377 https://doi.org/10.1016/j.jinf.2013.06.009
Purohit MR, Sviland L, Wiker H, Mustafa T (2017) Rapid and specific diagnosis of extrapulmonary tuberculosis by immunostaining of tissues and aspirates with anti-MPT64. Appl Immunohistochem Mol Morphol 25(4):282–288 https://doi.org/10.1097/PAI.0000000000000300
Islamov M, Sypabekova M, Kanayeva D, Rojas-Solorzano L (2017) CFD modeling of chamber filling in a micro-biosensor for protein detection. Biosensors (Basel) 7(4) https://doi.org/10.3390/bios7040045
Thakur H, Kaur N, Sabherwal P, Sareen D, Prabhakar N (2017) Aptamer based voltammetric biosensor for the detection of Mycobacterium tuberculosis antigen MPT64. Microchim Acta 184(7):1915–1922 https://doi.org/10.1007/s00604-017-2174-7
Parvin N, Jin Q, Wei Y, Yu R, Zheng B, Huang L, Zhang Y, Wang L, Zhang H, Gao M, Zhao H, Hu W, Li Y, Wang D (2017) Few-layer graphdiyne nanosheets applied for multiplexed real-time DNA detection. Adv Mater 29(18):1606755 https://doi.org/10.1002/adma.201606755
Cai J, Huang J, Ge M, Iocozzia J, Lin Z, Zhang KQ, Lai Y (2017) Immobilization of Pt nanoparticles via rapid and reusable electropolymerization of dopamine on TiO2 nanotube arrays for reversible SERS substrates and nonenzymatic glucose sensors. Small 13(19) https://doi.org/10.1002/smll.201604240
Ali MM, Li F, Zhang Z, Zhang K, Kang DK, Ankrum JA, Le XC, Zhao W (2014) Rolling circle amplification: a versatile tool for chemical biology, materials science and medicine. Chem Soc Rev 43(10):3324–3341 https://doi.org/10.1039/c3cs60439j
Zhang S, Wang K, Li KB, Shi W, Jia WP, Chen X, Sun T, Han DM (2017) A DNA-stabilized silver nanoclusters/graphene oxide-based platform for the sensitive detection of DNA through hybridization chain reaction. Biosens Bioelectron 91:374–379 https://doi.org/10.1016/j.bios.2016.12.060
Rafati A, Gill P (2014) Microfluidic method for rapid turbidimetric detection of the DNA of Mycobacterium tuberculosis using loop-mediated isothermal amplification in capillary tubes. Microchim Acta 182(3–4):523–530 https://doi.org/10.1007/s00604-014-1354-y
Takenaka M, Amino T, Miyachi Y, Ogino C, Kondo A (2017) Screening and evaluation of aptamers against somatostatin, and sandwich-like monitoring of somatostatin based on atomic force microscopy. Sensors Actuators B Chem 252:813–821 https://doi.org/10.1016/j.snb.2017.06.019
Zhou D, Xie G, Cao X, Chen X, Zhang X, Chen H (2016) Colorimetric determination of staphylococcal enterotoxin B via DNAzyme-guided growth of gold nanoparticles. Microchim Acta 183(10):2753–2760 https://doi.org/10.1007/s00604-016-1919-z
Casalini S, Dumitru AC, Leonardi F, Bortolotti CA, Herruzo ET, Campana A, de Oliveira RF, Cramer T, Garcia R, Biscarini F (2015) Multiscale sensing of antibody-antigen interactions by organic transistors and single-molecule force spectroscopy. ACS Nano 9(5):5051–5062 https://doi.org/10.1021/acsnano.5b00136
Lee H, Choi TK, Lee YB, Cho HR, Ghaffari R, Wang L, Choi HJ, Chung TD, Lu N, Hyeon T, Choi SH, Kim DH (2016) A graphene-based electrochemical device with thermoresponsive microneedles for diabetes monitoring and therapy. Nat Nanotechnol 11(6):566–572 https://doi.org/10.1038/nnano.2016.38
Chen Y, Li Y, Yang Y, Wu F, Cao J, Bai L (2017) A polyaniline-reduced graphene oxide nanocomposite as a redox nanoprobe in a voltammetric DNA biosensor for Mycobacterium tuberculosis. Microchim Acta 184(6):1801–1808 https://doi.org/10.1007/s00604-017-2184-5
Li Y, Pu Q, Li J, Zhou L, Tao Y, Li Y, Yu W, Xie G (2017) An “off-on” fluorescent switch assay for microRNA using nonenzymatic ligation-rolling circle amplification. Microchim Acta 184(11):4323–4330 https://doi.org/10.1007/s00604-017-2475-x
Iranmanesh M, Hulliger J (2017) Magnetic separation: its application in mining, waste purification, medicine, biochemistry and chemistry. Chem Soc Rev 46(19):5925–5934 https://doi.org/10.1039/c7cs00230k
Gao L, Zhuang J, Nie L, Zhang J, Zhang Y, Gu N, Wang T, Feng J, Yang D, Perrett S, Yan X (2007) Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat Nanotechnol 2(9):577–583 https://doi.org/10.1038/nnano.2007.260
Dendooven J, Ramachandran RK, Solano E, Kurttepeli M, Geerts L, Heremans G, Ronge J, Minjauw MM, Dobbelaere T, Devloo-Casier K, Martens JA, Vantomme A, Bals S, Portale G, Coati A, Detavernier C (2017) Independent tuning of size and coverage of supported Pt nanoparticles using atomic layer deposition. Nat Commun 8(1):1074 https://doi.org/10.1038/s41467-017-01140-z
Roh YH, Deng JZ, Dreaden EC, Park JH, Yun DS, Shopsowitz KE, Hammond PT (2016) A multi-RNAi microsponge platform for simultaneous controlled delivery of multiple small interfering RNAs. Angew Chem Int Ed Eng 55(10):3347–3351 https://doi.org/10.1002/anie.201508978
Yang Z, Zhao Y-P (2007) Adsorption of his-tagged peptide to Ni, cu and au (100) surfaces: molecular dynamics simulation. Engineering Analysis with Boundary Elements 31(5):402–409 https://doi.org/10.1016/j.enganabound.2006.07.012
Feng C, Bo B, Mao X, Shi H, Zhu X, Li G (2017) From interface to solution: integrating immunoassay with netlike rolling circle amplification for ultrasensitive detection of tumor biomarker. Theranostics 7(1):31–39 https://doi.org/10.7150/thno.16671
Gopinath SCB, Perumal V, Kumaresan R, Lakshmipriya T, Rajintraprasad H, Rao BS, Arshad MKM, Chen Y, Kotani N, Hashim U (2016) Nanogapped impedimetric immunosensor for the detection of 16 kDa heat shock protein against Mycobacterium tuberculosis. Microchim Acta 183(10):2697–2703 https://doi.org/10.1007/s00604-016-1911-7
Ji M, Cho B, Cho YS, Park SY, Cho SN, Jeon BY, Yoon BS (2014) Development of a quantitative sandwich enzyme-linked immunosorbent assay for detecting the MPT64 antigen of Mycobacterium tuberculosis. Yonsei Med J 55(3):746–752 https://doi.org/10.3349/ymj.2014.55.3.746
Thakur H, Kaur N, Sareen D, Prabhakar N (2017) Electrochemical determination of M. Tuberculosis antigen based on poly(3,4-ethylenedioxythiophene) and functionalized carbon nanotubes hybrid platform. Talanta 171:115–123 https://doi.org/10.1016/j.talanta.2017.04.063
Bai L, Chen Y, Bai Y, Chen Y, Zhou J, Huang A (2017) Fullerene-doped polyaniline as new redox nanoprobe and catalyst in electrochemical aptasensor for ultrasensitive detection of Mycobacterium tuberculosis MPT64 antigen in human serum. Biomaterials 133:11–19 https://doi.org/10.1016/j.biomaterials.2017.04.010
Acknowledgements
This research work was financially supported by the National Natural Science Foundation of China (No. 81672112).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOC 1.53 mb)
Rights and permissions
About this article
Cite this article
Gou, D., Xie, G., Li, Y. et al. Voltammetric immunoassay for Mycobacterium tuberculosis secretory protein MPT64 based on a synergistic amplification strategy using rolling circle amplification and a gold electrode modified with graphene oxide, Fe3O4 and Pt nanoparticles. Microchim Acta 185, 436 (2018). https://doi.org/10.1007/s00604-018-2972-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-018-2972-6