Abstract
Loop-mediated isothermal amplification (LAMP) eradicates the need of thermocycler in DNA amplification. Signals are usually obtained via fluorometry or turbidimetry, but such methods need improvement in order to become more effortless and reliable. The authors describe a set of six specific primers targeting the species-specific tlh gene of Vibrio parahaemolyticus which were used in accelerated LAMP reaction. Gold nanoparticles (AuNPs) were functionalized with streptavidin (Avidin-AuNPs), and engineered to signal the LAMP reaction. Two of the loop primers for LAMP were biotinylated and then can produce a DNA that can cause clusterization of Avidin-AuNPs based on the formation of avidin-biotin complex. This leads to a color change of the solution from red to blue. Amplification is completed within 30 min and can be visually detected within 5 min. The detection limit of the method is found to be 8.6 cfu per reaction. This visual detection scheme does not require any fluorescent reagents and detection instruments. Conceivably, the method has a wide scope because such Avidin-AuNPs can be used as nanoprobes for a variety of other LAMP products. This rapid and universal strategy holds promise in point of care testing and food testing, particularly in resource-limited regions.

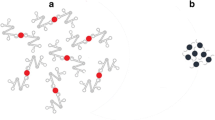
Six specific primers (two of them are biotinylated) were used to realize the accelerated Loop-Mediated Isothermal Amplification. Streptavidin modified gold nanoparticles (Avidin-AuNPs) cluster on the DNA products, leading to the apparent change of color from red to blue, which is readily identified even by unaided eye.



Similar content being viewed by others
Change history
28 December 2017
The published version of this article, unfortunately, contained error. Modifications have been made to the Abstract, Introduction, Results and discussion, and Acknowledgements section. The original article has been corrected.
References
Jones J, Lydon K, Kinsey T, Friedman B, Curtis M, Schuster R, Bowers J (2017) Effects of ambient exposure, refrigeration, and icing on Vibrio vulnificus and Vibrio parahaemolyticus abundances in oysters. Int J Food Microbiol 253:54–58
Xiang G, Pu X, Jiang D, Liu L, Liu C, Liu X (2013) Development of a real-time resistance measurement for vibrio parahaemolyticus detection by the lecithin-dependent hemolysin gene. PLoS One 8(8):e72342
Martinez-Urtaza J, Lozano-Leon A, Viña-Feas A, de Novoa J, Garcia-Martin O (2006) Differences in the API 20E biochemical patterns of clinical and environmental Vibrio parahaemolyticus isolates. FEMS Microbiol Lett 255(1):75–81
Zeng J, Wei H, Zhang L, Liu X, Zhang H, Cheng J, Ma D, Zhang X, Fu P, Liu L (2014) Rapid detection of Vibrio parahaemolyticus in raw oysters using immunomagnetic separation combined with loop-mediated isothermal amplification. Int J Food Microbiol 174:123–128
Yi M, Ling L, Neogi SB, Fan Y, Tang D, Yamasaki S, Shi L, Ye L (2014) Real time loop-mediated isothermal amplification using a portable fluorescence scanner for rapid and simple detection of Vibrio parahaemolyticus. Food Control 41:91–95
Duan N, Shen M, Wu S, Zhao C, Ma X, Wang Z (2017) Graphene oxide wrapped Fe3O4@ Au nanostructures as substrates for aptamer-based detection of Vibrio parahaemolyticus by surface-enhanced Raman spectroscopy. Microchim Acta 184(8):2653–2660
Teng J, Ye Y, Yao L, Yan C, Cheng K, Xue F, Pan D, Li B, Chen W (2017) Rolling circle amplification based amperometric aptamer/immuno hybrid biosensor for ultrasensitive detection of Vibrio parahaemolyticus. Microchim Acta 184(9):3477–3485
Notomi T, Okayama H, Masubuchi H, Yonekawa T, Watanabe K, Amino N, Hase T (2000) Loop-mediated isothermal amplification of DNA. Nucleic Acids Res 28(12):e63–e63
Mori Y, Notomi T (2009) Loop-mediated isothermal amplification (LAMP): a rapid, accurate, and cost-effective diagnostic method for infectious diseases. J Infect Chemother 15(2):62–69
Kaneko H, Kawana T, Fukushima E, Suzutani T (2007) Tolerance of loop-mediated isothermal amplification to a culture medium and biological substances. J Biochem Biophys Methods 70(3):499–501
Tomita N, Mori Y, Kanda H, Notomi T (2008) Loop-mediated isothermal amplification (LAMP) of gene sequences and simple visual detection of products. Nat Protoc 3(5):877
Notomi T, Mori Y, Tomita N, Kanda H (2015) Loop-mediated isothermal amplification (LAMP): principle, features, and future prospects. J Microbiol 53(1):1–5
Ayukawa Y, Hanyuda S, Fujita N, Komatsu K, Arie T (2017) Novel loop-mediated isothermal amplification (LAMP) assay with a universal QProbe can detect SNPs determining races in plant pathogenic fungi. Sci Rep 7(1):4253. https://doi.org/10.1038/s41598-017-04084-y
Boehme CC, Nabeta P, Henostroza G, Raqib R, Rahim Z, Gerhardt M, Sanga E, Hoelscher M, Notomi T, Hase T (2007) Operational feasibility of using loop-mediated isothermal amplification for diagnosis of pulmonary tuberculosis in microscopy centers of developing countries. J Clin Microbiol 45(6):1936–1940
Goto M, Honda E, Ogura A, Nomoto A, Hanaki KI (2009) Colorimetric detection of loop-mediated isothermal amplification reaction by using hydroxy naphthol blue. BioTechniques 46(3):167–172. https://doi.org/10.2144/000113072
Rafati A, Gill P (2015) Microfluidic method for rapid turbidimetric detection of the DNA of Mycobacterium tuberculosis using loop-mediated isothermal amplification in capillary tubes. Microchim Acta 182(3–4):523–530
Tian B, Ma J, Zardán Gómez de la Torre T, Bálint A, Donolato M, Hansen MF, Svedlindh P, Strömberg M (2016) Rapid Newcastle disease virus detection based on loop-mediated isothermal amplification and Optomagnetic readout. Acs Sensors 1(10):1228–1234
Martin A, Grant KB, Stressmann F, Ghigo J-M, Marchal D, Limoges B (2016) Ultimate single-copy DNA detection using real-time electrochemical LAMP. Acs Sensors 1(7):904–912
Nagamine K, Hase T, Notomi T (2002) Accelerated reaction by loop-mediated isothermal amplification using loop primers. Mol Cell Probes 16(3):223–229
Zhang H, Wang S, Chen Z, Ge P, Jia R, Xiao E, Zeng W (2017) A turn-on fluorescent nanoprobe for lead(II) based on the aggregation of weakly associated gold(I)-glutathione nanoparticles. Microchim Acta 184(10):1–7
Li M, Shi L, Xie T, Jing C, Xiu G, Long Y-T (2017) An ultrasensitive Plasmonic Nanosensor for aldehydes. ACS Sensors 2(2):263–267
Yu R-J, Sun J-J, Song H, Tian J-Z, Li D-W, Long Y-T (2017) Real-time sensing of O-Phenylenediamine oxidation on gold nanoparticles. Sensors 17(3):530
Han X, Liu Y, Yin Y (2014) Colorimetric stress memory sensor based on disassembly of gold nanoparticle chains. Nano Lett 14(5):2466–2470. https://doi.org/10.1021/nl500144k
Shi H-y, Yang L, Zhou X-y, Bai J, Gao J, Jia H-x, Li Q-g (2017) A gold nanoparticle-based colorimetric strategy coupled to duplex-specific nuclease signal amplification for the determination of microRNA. Microchim Acta 184(2):525–531
Nordstrom JL, Vickery MCL, Blackstone GM, Murray SL, DePaola A (2007) Development of a multiplex real-time PCR assay with an internal amplification control for the detection of Total and pathogenic Vibrio parahaemolyticus bacteria in oysters. Appl Environ Microbiol 73(18):5840–5847. https://doi.org/10.1128/aem.00460-07
Haiss W, Thanh NT, Aveyard J, Fernig DG (2007) Determination of size and concentration of gold nanoparticles from UV− Vis spectra. Anal Chem 79(11):4215–4221
Jazayeri MH, Amani H, Pourfatollah AA, Pazoki-Toroudi H, Sedighimoghaddam B (2016) Various methods of gold nanoparticles (GNPs) conjugation to antibodies. Sens Bio-Sens Res 9:17–22. https://doi.org/10.1016/j.sbsr.2016.04.002
National food safety standard food microbiological examination: Vibrio parahaemolyticus (2013) China standard protocols, GB4789.7-2013. National health and family planning commission of the PRC, the People’s Republic of China
Zhong Q, Tian J, Wang B, Wang L (2016) PMA based real-time fluorescent LAMP for detection of Vibrio parahaemolyticus in viable but nonculturable state. Food Control 63:230–238
Wang R, Xiao X, Chen Y, Wu J, Qian W, Wang L, Liu Y, Ji F, Wu J (2017) A loop-mediated, isothermal amplification-based method for visual detection of Vibrio parahaemolyticus within only 1 h, from shrimp sampling to results. Anal Methods 9(11):1695–1701
Yamazaki W, Ishibashi M, Kawahara R, Inoue K (2008) Development of a loop-mediated isothermal amplification assay for sensitive and rapid detection of Vibrio parahaemolyticus. BMC Microbiol C7–163 8(1):1–7. https://doi.org/10.1186/1471-2180-8-163
Chen S, Wang F, Beaulieu JC, Stein RE, Ge B (2011) Rapid detection of viable salmonellae in produce by coupling propidium monoazide with loop-mediated isothermal amplification. Appl Environ Microbiol 77(12):4008–4016
Ahmad F, Stedtfeld RD, Waseem H, Williams MR, Cupples AM, Tiedje JM, Hashsham SA (2017) Most probable number-loop mediated isothermal amplification (MPN-LAMP) for quantifying waterborne pathogens in< 25min. J Microbiol Methods 132:27–33
Wang L, Shi L, Su J, Ye Y, Zhong Q (2013) Detection of Vibrio parahaemolyticus in food samples using in situ loop-mediated isothermal amplification method. Gene 515(2):421–425. https://doi.org/10.1016/j.gene.2012.12.039
Gao H, Lei Z, Jia J, Wang S, Chen Y, Sun M, Liang C (2009) Application of loop-mediated isothermal amplification for detection of Yersinia enterocolitica in pork meat. J Microbiol Methods 77(2):198–201
Acknowledgements
The authors acknowledge the financial support provided by the Yangfan project (14YF1408100) from Science and Technology Commission of Shanghai Municipality – PR China and the special research fund for the national non-profit institutes (East China Sea Fisheries Research Institute) (No. 2014 T05). E.K.F. thanks the World Academy of Sciences (TWAS) under the Grant No. 16-510 RG/CHE/AF/AC_G–FR3240293301 for its financial support.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
There is no conflict of interest about this article.
Additional information
The original version of this article was revised: The published version of this article, unfortunately, contained error. Modifications have been made to the Abstract, Introduction, Results and discussion, and Acknowledgments section.
A correction to this article is available online at https://doi.org/10.1007/s00604-017-2617-1.
Electronic supplementary material
ESM 1
(PDF 1.57 mb)
Rights and permissions
About this article
Cite this article
Kong, C., Wang, Y., Fodjo, E.K. et al. Loop-mediated isothermal amplification for visual detection of Vibrio parahaemolyticus using gold nanoparticles. Microchim Acta 185, 35 (2018). https://doi.org/10.1007/s00604-017-2594-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-017-2594-4