Abstract
Nucleic acids are prominent biomarkers for diagnosing infectious pathogens using nucleic acid amplification techniques (NAATs). PCR, a gold standard technique for amplifying nucleic acids, is widely used in scientific research and diagnosis. Efficient pathogen detection is a key to adequate food safety and hygiene. However, using bulky thermal cyclers and costly laboratory setup limits its uses in developing countries, including India. The isothermal amplification methods are exploited to develop miniaturized sensors against viruses, bacteria, fungi and other pathogenic organisms and have been applied for in situ diagnosis. Isothermal amplification techniques have been found suitable for POC techniques and follow WHO’s ASSURED criteria. LAMP, NASBA, SDA, RCA and RPA are some of the isothermal amplification techniques which are preferable for POC diagnostics. Furthermore, methods such as WGA, CPA, HDA, EXPAR, SMART, SPIA and DAMP were introduced for even more accuracy and robustness. Using recombinant polymerases and other nucleic acid-modifying enzymes has dramatically broadened the detection range of target pathogens under the scanner. The coupling of isothermal amplification methods with advanced technologies such as CRISPR/Cas systems, fluorescence-based chemistries, microfluidics and paper-based sensors has significantly influenced the biosensing and diagnosis field. This review comprehensively analyzed isothermal nucleic acid amplification methods, emphasizing their advantages, disadvantages and limitations.
Similar content being viewed by others
Introduction
DNA, a unique molecule in each organism, signifies a promising recognition molecule for identifying living organisms. Viruses are also essential molecules, as they play an important role in our life. The identification of viruses is critical to check substantial economic losses. Altogether, DNA and RNA are both important biomarkers for diagnostics and biological studies. This is possible as they show complementarity binding. In addition, DNA and RNA show high affinity toward ions, signaling molecules like hormones and proteins, and even whole cells (Yan et al. 2014).
Many molecular diagnostic methods have drastically evolved to sense nucleic acids in past decades (Martzy et al. 2019). Most of these molecular methods are based on nucleic acid amplification (NAA). NAA are of two types: (i) thermocycling amplification and (ii) isothermal amplification; the former is extensively used with a number of variations, whereas the latter is new and offers plenty of scope (Ignatov et al. 2014). For NAA, the prime requirement is access to DNA or RNA. The process can be done separately: performing the isolation, amplification and detection or combining the isolation, amplification and detection in one step. The latter is more challenging, but it is a method of choice for biosensor development that is about to enter the market in the coming years and will replace the multiple-step process (Craw and Balachandran 2012; Ho et al. 2018).
The commercial NAA-based detection kit primarily uses polymerase chain reaction (PCR) to amplify and detect specific DNA/RNA targets. Kary Mullis in 1983 first introduced PCR, which became the most extensively used technique for NAA. It can produce billion-fold copies of nucleic acid with the help of enzymatic reactions carried out in a thermocycler (Gill and Ghaemi 2008; Prasad et al. 2011).
The first step in PCR is denaturation, which is carried out at a temperature of 95° C and results in the separation of the two strands of DNA into single strands, serving as a template for the next steps. The primers and other required enzymes get attached at the 5ʹ end of the template strand in the second annealing step. Sequence information is essential for GC content and primer designing. The third step is in which primers are extended with the help of DNA polymerases on the template strand from 5ʹ to 3ʹ direction to produce copies of the target (Rajalakshmi 2017). PCR has limitations in resource-compromised settings, such as requiring trained technicians, costly equipment and sample transportation to the laboratory (Fakruddin et al. 2013). Accordingly, the World Health Organization (WHO) has summarized the requirements for point-of-care (POC) analysis in their ASSURED guidelines (affordable, sensitive, specific, user-friendly, robust, and rapid, easy to use and deliverable to people in need) (Martzy et al. 2019).
In the past three decades, several platforms of isothermal amplification techniques (IAT) were developed to meet the minimum requirements of ASSURED guidelines. These techniques are fast, reliable and performed in a thermostat water bath/dry bath with minimum expenditure (Oriero et al. 2015). These IAT use strand displacement activity to carry out NAA without thermocyclers, thus eliminating the temperature cycling step (Zhao et al. 2015). The most widely used IAT include loop-mediated isothermal amplification (LAMP), strand displacement amplification (SDA), nucleic acid sequence-based amplification (NASBA), helicase-dependent amplification (HDA), rolling circle amplification (RCA), etc. Various kits based on different IAT have been developed, making diagnosis easier for unskilled personnel, as summarized in Table 1.
Isothermal amplification techniques
Unlike PCR, IAT is a simple technique that amplifies DNA at a constant temperature. The amplification process is swift, as it does not involve additional denaturation steps in between. The credit goes to DNA polymerases, RNA polymerases and other nucleic acid-modifying enzymes, making this process happen in isothermal conditions. Bst DNA polymerase (utilized in LAMP) was first isolated by Stenesh and Roe from a moderately thermophilic bacteria Bacillus stearothermophilus having optimum growth temperature of 60°–70° C (Aliotta et al. 1996) and shows maximum activity close to 65° C. The large fragment of Bst DNA polymerase shows 5′→3′ polymerization along with strand displacement activity, making it capable of dissociating hydrogen bonds between the two strands of DNA.
One of the key parameters for evaluating the effect of an amplification reaction is its ‘digital efficiency’, i.e., the percentage of templates that are successfully amplified from the total template pool. To increase this efficiency for nucleic acid amplification, many improvements were made. IAT produces high copy number in minimum time at constant temperature (Fakruddin et al. 2013). NASBA is an isothermal amplification technique faster than PCR but limited by RNA secondary structure, which complicates primer designing and multiplexing. Further multiple heating and lateral entry of amplifying enzyme make NASBA clumsy. However, LAMP does not require pure template/sample preparation and the organism serves as an analyte. This technique proved to be a promising alternative to other IAT, as it can be performed at remote locations at POC sites without trained technicians and sophisticated laboratory (Zanoli and Spoto 2013). The isothermal amplification techniques can be classified based on DNA replication, enzyme-based DNA digestion or reaction kinetics. IATs are expanded to detect different targets, including proteins, cells, small molecules and ions (Niemz et al. 2011). The combination of isothermal amplification and nanotechnology has also become a point of interest. Isothermal amplification can provide a large number of nucleic acids as materials for developing complex nucleic acid nanostructures and nucleic acid hydrogels with wide applications in biosensing, bioimaging and nanomedicine. Use of molecular beacons (MBs) is also considered in many isothermal amplification assay developments. These single-stranded oligonucleotide hybridization probes, which form a stem loop structure, are used for nucleic acid detection, which was introduced in the 1990s. Both arms of the stem loop are labeled with a fluorophore at one end and a quencher at another end, so when both arms are in proximity, fluorescence is dimmed, but when it is attached to a complementary strand, the stem loop opens up, resulting in the restoration of fluorescence (Yan et al. 2014).
Recent developments in microfabrication have led to the association of these methods with microfluidic chips, capillary platforms and paper-based devices. Microfluidic methods helped in shorter amplification reaction times and reduced reaction volume. It has also enabled digital quantification as an alternative to real-time (kinetic) quantification (Zhang et al. 2019a, b, c, d). The recent pandemic of SARS-CoV 2 has led the whole world to a serious concern for the need for rapid, sensitive and onsite diagnostic techniques for COVID-19. A comparative study of different IAT for COVID-19 diagnosis can be found in Tran et al. (2020).
Here, we will discuss various IATs described in Table 2 and Fig. 1. Few methods are complex and are not adopted in routine use.
Loop-mediated isothermal amplification (LAMP)
LAMP was developed for sequence-specific isothermal DNA amplification suitable for clinical diagnostics. The amplified product includes multiple different sizes of DNA structures, which are identified by a smear of banding pattern on gel electrophoresis (Yao et al. 2016). It uses Bst DNA polymerase isolated from Bacillus stearothermophilus to carry out amplification. This isothermal amplification process produced approximately 109 copies in 30–60 min at 60°–65° C. This technique requires four primers: backward inner primer (BIP), forward inner primer (FIP), forward outer primer (F3) and backward outer primer (B3) (Notomi et al. 2000). To accelerate the amplification process, two additional primers named forward and reverse loop primers can be incorporated into the reaction (Nagamine et al. 2002). During the initial phase, strand displacement activity leads to the formation of a dumbbell-shaped structure, while in the secondary phase, cycling amplification is carried out which requires only two inner primers. The result produces stem loop DNA, which contains several repeats of target sequence and cauliflower-like multiple loop structure. The detection efficiency of LAMP can be improved by additional loop primers (LF/LB) (Fig. 2) (Notomi et al. 2015). This specificity has allowed the insoluble pyrophosphate reaction by-product to be employed in a turbidimetric detection strategy for either qualitative visual indication or real-time quantitative turbidimetry (Yang et al. 2018a, b, c). LAMP technique can also be used for RNA detection by the implementation of reverse transcriptase enzyme, which transcripts RNA into cDNA (RT-LAMP), dependent on both RTase and Bst polymerase enzyme.
Schematic representation of the LAMP reaction. (I) Formation of dumbbell-shaped structure that starts from the FIP and BIP primers. (II) Cycling amplification of the dumbbell-shaped structure results in a stem–loop structure, which then (III) forms a cauliflower-like structure in the elongation and recycling step, resulting in different sizes of DNA amplicons producing a ladder pattern in agarose gel electrophoresis (reproduced with permission by Notomi et al. (2000), Copyright
New findings and modifications are ongoing for the development of better LAMP techniques, such as modifications in components, efficiency and detection methods. LAMP has been extended to different field of microfluidics such as classical microfluidics (single gene detection, multi-gene detection and RT-LAMP), paper-based microfluidics (nitrocellulose-based FTA and glass-based FTA) and digital LAMP. LAMP technique is currently used in different ways for the detection of LAMP amplicons such as gel electrophoresis, naked eye detection, turbidimetric, colorimetric, bioluminescence, fluorescence, electrochemical sensors/chips, ELISA and lateral flow dipstick (LFD) (Zhong and Zhao 2017). Shang et al. in (2018) classified LAMP techniques in their review article based on their detection methods, i.e., (i) end point detection methods (turbidity, dyes, electrochemical, electrophoresis and immunoassay) and (ii) real-time detection methods (RT-turbidity, RT-fluorescence, RT-electrochemical and surface plasmon resonance). Shang et al. (2018) also described the efficiency, sensitivity, and specificity of different detection methods integrated with LAMP. Other techniques used for detection include multiplex analysis (Jang et al. 2021), droplet digital LAMP (Fan et al. 2022), nano-Au probe (Habiburrahman et al. 2021), field effect transistors (Park et al. 2021), magnesium pyrophosphate precipitation (Mori et al. 2004), Cu(OH)2 precipitation (Kanchanaphum 2018 etc. which are current areas of research (Fig. 3).

© 2021, American Chemical Society). (II) Droplet generation by inkjet printer. a–c Droplet generation from inkjet printer and its microscopic view (reprinted with permission from Fan et al. (2022), Copyright © 2022, MDPI, Basel, Switzerland). (III) Colorimetric detection of Mycobacterium tuberculosis from sputum sample using Au-NP. (1–3) DNA extraction and amplification through LAMP. (4) Preparation of Au-NP using HAuCl4 and sodium citrate tribasic dihydrate. (5) Dia-ultrafiltration of the prepared AuNP, (6) MgSO4 and MgCl2 are added to induce aggregation in the absence of DNA. Detection through colorimetric method (reprinted with permission from Habiburrahman et al. (2021), Copyright © 2021, Journal of Infection in Developing Countries). (IV) Design of a turbidimeter for MgPO4 quantification. (A) Turbidimeter consists of eight light emitting diodes and photodiodes and eight sample positions. (B) Orthographic view of the turbidimeter (reprinted with permission from Mori et al. (2004), Copyright © 2003 Elsevier B.V.)
(I) Schematic diagram of SARS-CoV 2 detection using crumpled graphene field effect transistor (cgFET) device. A Genome target site of SARS-CoV 2. B Workflow of sample collection and isothermal amplification for detection. C Crumpled graphene (cg) is used as a sensing material for FET and the chamber is used for isolating virus samples (reprinted with permission from Park et al. (2021), Copyright
The LAMP technique has been used mainly in the diagnostics of pathogens. Since its introduction in 2000, many researchers have worked to improve and modify this technique (Table 3). LAMP technology was used to detect Salmonella species for initial analysis and food applications (Hara-kudo et al. 2005). Since then, many LAMP assays have been developed and employed with different primer designs for the detection of non-typhoidal Salmonella in various applications as well as the detection of other different pathogens by designing specific primers (Table 4) (Ohtsuka et al. 2005; Zhong and Zhao 2017). Panek and Frąc (2019) designed a LAMP technique for detection of Talaromyces flavus, a soilborne fungus, which is responsible for contamination of fruits. They performed a test with 5 strains of T. flavus and 35 other fungal isolates. The results showed the LOD of 1 fg of genomic DNA and approximately 64 ascospores in 1 g of strawberry fruit and soil sample. Takano et al. (2019) developed a novel LAMP method for rapid identification of β-lactamase-producing Pseudomonas aeruginosa strain. They developed two types of primers which target bla genes (GES-LAMP) and point mutation target site (Carba-GES-LAMP). Limit of detection of novel LAMP assay was evaluated using DNA spike in clinical samples to obtain better sensitivity and specificity. The result concluded that this novel method was better in terms of sensitivity which was tenfold more than conventional PCR. Nzelu et al. (2019) tested LAMP amplification technique for detecting sensitivity and specificity toward leishmaniasis-causing protozoan parasites. The test resulted in 80–100% sensitivity and 94%–100% specificity compared to other methods. This resulted in the discovery of an efficient leishmania detection method as POC analysis. Avelar et al. (2019) was able to develop a novel LAMP technology for detection of visceral leishmaniasis, a tropical disease. They targeted the K-26 antigen-coding gene of the L. donovani complex. They collected 219 blood samples which included VL and non-VL samples, which are tested with parasitological and serological tests as a reference to calculate the diagnostic accuracy. The result concluded that this technique was better than kDNA Leishmania PCR and is specific for L. donovani as it was able to detect 1 fg of L. infantum complex in sample with 100 parasites/ml in just 60 min. This technique proved to be a rapid identification technology for visceral leishmaniasis detection. Mohon et al. (2019) described an ultrasensitive loop-mediated isothermal amplification for the detection of malarial parasites from dried blood spots and whole blood cells. They modified P. falciparum (Pf)-specific primers and compared with a previously described set of genus-specific primers of Plasmodium (Pan). He et al. (2019) compared the LAMP technique with HRCA (hyperbranched rolling circle amplification) technique for the rapid detection of lethal Amanita species. They designed ten sets of LAMP primers using Primer Explorer V5 and ten padlock probes (PLPs) were designed according to the previously described method. They reported that the LAMP technique was able to discriminate between introcalade species but not intracalade, while HRCA was able to discriminate both types of species. The LOD reported for both the technique was 10 pg for LAMP and 1 pg for HRCA of genomic DNA copies per reaction. Therefore, they concluded that both the technique was able to detect Amanita species rapidly in comparison of conventional PCR assays.
Dye-based detection technique or naked eye detection technique is easier to use for the rapid detection of pathogens using LAMP. Many researchers till now have worked to test different types of dyes for the detection of various pathogens. Ding et al. in (2015) reported a mixed dye containing HNB and SYBR Green I, which could improve the detection sensitivity and avoid the empirical pre-set of cutoff intensity values, making the result more accurate. Lee et al. in (2017) used LAMP for the detection of Middle East respiratory syndrome coronavirus (MERS-CoV). The primers were designed according to the nucleocapsid region of the virus and employed in RT-LAMP (One pot RT-LAMP) technology. The technique detected four genome copies of the virus in 60 min. They used Eva-green dye instead of SYBR Green dye, which is more compatible with DNA polymerase. Nguyen et al. (2019) used cheap metal indicators (such as hydroxynaphthol blue or Eriochrome Black T) instead of using the high-priced fluorescence probes, for evaluating the success or failure of LAMP reaction. They demonstrated how the shift of specific absorbance peaks of EBT dye can be utilized for the development of an EBT-based quantitative LAMP reaction. The initial state of the LAMP mixture was violet, which shows the maximum peak at 570 nm, while the final color of the LAMP mixture after a successful LAMP reaction was sky blue, which displayed the maximum peak at 640 nm. Therefore, they evaluated the LAMP reaction by just calculating the shift between the two peaks.
Liu et al. in (2018) compared three assays of LAMP technology for the detection of the SVCV virus. The three assays (SYBR dye, lateral flow dipstick and agarose gel electrophoresis) were used to conclude which assay was better. The result concluded that the LFD-LAMP assay could be employed for the detection of SVCV virus in comparison to others for field-based detection. The LFD-LAMP detected 860 fg of genome copies of cDNA per reaction. Rohatensky et al. in (2018) reported the efficiency of LAMP in the detection of high-risk subtypes of HPV responsible for causing oropharyngeal squamous cell carcinoma (OPSCC). They compared this with their previous study in which they performed HPV LAMP assay with DNA purification. In this study, they did not purify the DNA and designed the primers for HPV 31 and 35. For HPV 16 and 18 subtypes, they used already established primers (Saetiew et al. 2011). The result indicated an excellent clinically relevent assay that is rapid, cost-effective and easy readout system.
Velders et al. (2018) developed an Arduino-based DNA detection technology for LAMP amplification. According to them, the previous LAMP technique was not stable to be performed for a long time and with heavy DNA samples, as well as it was hard to operate for untrained personnel. So, they developed KISS (keep it simple, silly) technology for the detection of DNA through LAMP. The new modified version was battery operated and easy to use with proper indications, which made it much easier for untrained personnel to operate. Schneider et al. (2019) developed a mathematical model for LAMP to avoid false positive results by predicting DNA banding patterns and size of DNA based on LAMP structure. This mathematical model emerged as a new breakthrough for predicting the amplification results, so that the LAMP technique can be performed easily without consuming time.
LAMP on a chip (LOAC) is a term for different types of POC devices based on microfluidics, paper-based and digital variants (dLAMP). The capability of LOAC depends on simple hardware, sample preparation and loading steps and its compatibility with LAMP (Zhang et al. 2019a, b, c, d). A magnetic bead-based LAMP assay was developed for the single gene detection of methicillin-resistant Staphylococcus aureus. Nucleotide-coated magnetic beads, washing buffer and reaction mixture were loaded in chambers of three different chips and then subjected to LAMP. Results were analyzed through a spectrophotometer and the LOD was approximately 10 fg/µL (Wang et al. 2011). Multi-gene detection was performed by fabricating an octopus-like multi-channel chip, and the sample was loaded in each well using capillary force. LOD obtained was less than 10 copies/µL. Although these devices are efficient for pathogen detection, the use of pumps for capillary force increases the cost and complexity of the system (Fang et al. 2011). Shang et al. (2018), in their review, summarized the use of LAMP technology with different microfluidics techniques. Chen et al. in (2017) developed a PDMS microchip, in which each reaction chamber contains low-melting point agarose and all LAMP reagents except the DNA samples. This microchip can simultaneously detect four pathogens. Calcein dye was used to detect the amplicons with a limit of detection (LOD) of three copies per liter for Escherichia coli. Pang et al. in (2018) found that mixed dye was very suitable to be applied on microchips, so a self-priming compartmentalization (SPC) microchip was developed to detect Vibrio parahaemolyticus by applying a mixed-dye-loaded LAMP reaction.dLAMP was first developed by Gansen et al. (2012) using the properties of dPCR, but with more advantages such as absolute quantitative determination of nucleic acid, higher accuracy and sensitivity and decreased complexity of instruments, making it useful in developing POCT (point-of-care techniques). However, dLAMP has a lower digital efficiency which causes inaccurate quantification. To tackle this problem, a prehybridization-induced enhancement strategy was applied to enhance its digital efficiency up to 2–40 times (Xue et al. 2021). Other techniques for dLAMP include gLAMP (gel LAMP), which uses a gel matrix prepared by mixing LAMP mixtures in polyethylene glycol hydrogel, whereas bead-based LAMP is developed by using magnetic beads to capture DNA and then emulsifying with LAMP mixtures. Both gLAMP and bead-based LAMP have some advantages and disadvantages: gLAMP is much simpler in terms of fabrication, unlike microfluidic dLAMP, but it can detect only a low number of copies, whereas bead-based LAMP can quantify an absolute number of copies (5–150 copies per µL), but lower than microfluidics dLAMP (25–2.5 × 107 copies per µL) (Huang et al. 2018). Commercial membrane-based dLAMP uses a commercial porous membrane allowing to compartmentalize LAMP mixtures in the pores and each pore functions as a single nanoreactor. This microfluidic-free device can detect up to 11–1.1 × 105 copies per µL (Lin et al. 2019), leading to the elimination of cost used in fabricating a PDMS or plastic-based microfluidic device. Microfluidics is one of the important aspects for a POC device for which dLAMP has been used in the development of chamber-based microfluidic devices and droplet microfluidic devices. Chamber-based devices have compartments where LAMP mixtures are stored, while droplet microfluidics compartmentalizes LAMP mixtures through emulsification (Yuan et al. 2020). Droplet microfluidics devices have the upper hand compared to chamber-based devices such as automatization, low costs and adjustable reaction volume (Yuan et al. 2020). Paper-based microfluidics have an advantage over classical microfluidics systems such as user friendliness, low cost, simple design and robustness. The most common materials used for paper-based microfluidic systems are nitrocellulose-based FTA or glass beads (Zhang et al. 2019a, b, c, d). In recent years, paper origami-based microfluidic chip technique has evolved widely and become an area of interest for researchers working on the improvement of DNA amplification technologies. Xu et al. in (2016) reported a diagnostic platform that uses paper folding (origami) to integrate the different blood sample preparation steps that are required for LAMP onto a paper microfluidic device. The paper substrate is inexpensive and has a high surface-to-volume ratio, and paper-based microfluidic devices have emerged as a low-cost and easy-to-use platform. Using poly(methyl methacrylate) (PMMA), a cassette was developed consisting of a single amplification chamber connected to the inlet for the detection of HIV. The FTA membrane was incorporated into the chamber for RNA isolation, concentration and purification. This method was able to achieve an LOD of < 10 HIV virions (Liu et al. 2011). A magnetic strip coupled with an FTA-based disc was fixed between two magnetic layers. This contains serial ports for sampling, washing, amplification and detection. The probe's fluorescence was detected using a handheld ultraviolet source, achieving an LOD of five cells (Connelly et al. 2015). Lateral flow assay (LFA) is a type of simple and low-cost POC technique which is used in the field for diagnostic purpose without the requirement of large equipment. This LFA can be coupled with LAMP for faster diagnosis of various diseases. LFAs are based on specific antibodies and bacteria. However, this method has a very low sensitivity detection and can detect only a high pathogen concentration (105 CFU per mL) (Gong et al. 2021; Kim et al. 2022). In an experiment, NALFA (nucleic acid lateral flow assay) is used for multiplex detection of Salmonella and Staphylococcus aureus. NALFA strips were designed using NC membrane, backing card and absorbent pad for easier capillary movement. The developed assay detected 1.6 CFU of Salmonella sp. (Fig. 4) (Kim et al. 2022). Lateral flow dipstick assay was used to detect Mycoplasma ovipneumoniae using LAMP. This method uses a biotin LAMP product hybridized with a digoxin-labeled DNA probe that is complexed with a gold-labeled anti-digoxin antibody. Minimum detection limit of this method was found to be 1 × 102 CFU/mL indicating that this method has higher sensitivity, specificity and is easier to operate (Zhang et al. 2019a, b, c, d). A multiplex LFA–LAMP coupled with neutravidin-tagged gold nanoparticles was developed for the detection of sea and seb genes of Staphylococcus aureus. Digoxigenin and biotin were used to label sea genes, while FITC (fluorescein thiocyanate) was used for seb genes. LOD was found to be 103 CFU per mL, while in real samples it was 102 CFU per mL resulting in a faster and more efficient method for the detection of S. aureus (Yin et al. 2016). E. coli was detected using an LFA–LAMP-based assay labeled with a digoxygenin-labeled LAMP amplicon and biotinylated probe. LOD was found to be around 103 CFU per mL showing that this method has ten times higher sensitivity than PCR and will be useful in detecting real samples (Kim and Oh 2019).
Schematic diagrams of LAMP reaction and working of NALFA strips. The flowchart shows: sample collection and thermal lysis for DNA extraction for LAMP. Amplified DNA was added to the NALFA strips to detect Salmonella and Staphylococcus aureus (reprinted with permission from Kim et al. (2022), Copyright
The recent outbreak of novel coronavirus SARS-CoV 2 has threatened global health and is a serious cause of concern. New diagnostic techniques using LAMP have been introduced for rapid and sensitive detection of coronavirus which can be used on POC sites. In a study, colorimetric-based RT-LAMP was able to detect as low as 100 copies of SARS-CoV 2 RNA (Park et al. 2020). In another study, a technique named iLACO (isothermal LAMP-based method for COVID-19) was developed to amplify the fragment of the ORF1ab gene. Species specificity was assured by comparing the genome of 11 related virus using BLAST. This colorimetric RT-LAMP-based coronavirus detection technique achieved LOD of as low as ten copies, which was much more sensitive compared to the RT-qPCR technique (Yu et al. 2020). A nanoparticle-based lateral flow biosensor was developed using multiplex RT-LAMP for diagnosis of COVID-19. Two set of LAMP primers for ORF1ab and N-nucleoprotein gene were used for simultaneous amplification of in a single tube reaction. The primer were labeled with ITC/digoxin and biotin, which were determined by LFB. Accumulation of nanoparticles leads to the visualization of a crimson band showing multiplex analysis of ORF1ab gene and N gene. LOD was found to be 12 copies per reaction with an analytical sensitivity of 100% for SARS-CoV 2 (Zhu et al. 2020). A paper-based assay was developed for the detection of Candida albicans by our group (Sudarsan et al. 2023). Herein, DNA was amplified using LAMP and compacted with CTAB and PEG 8000. The compaction of DNA has increased the fluorescence signal up to twofold. The impact of an enhanced signal can also be attributed to LAMP, as it can efficiently amplify DNA with high yield in a short duration with high specificity. However, non-specific results in LAMP could occur due to dimer hybridization of primers, due to high concentration of Bst DNA polymerase. The problem of non-specific amplification of LAMP may be reduced by the addition of 1% pullulan in the reaction (Xueqin Gao et al. 2019b, a). Becherer et al. in (2020) described in their review about different technique developed using LAMP based on their homogeneity and heterogeneity. This is further divided into particle-based and particle-free detection, which is further classified into types of detection techniques. LAMP has become a promising approach in the field of clinical diagnostics. LAMP kits for tuberculosis and malaria are quite developed and already commercialized. (available in Human Diagnostic Worldwide; https://www.human.de/products/molecular-dx/) (Diego et al. 2019). Besides the several advantages, LAMP has few limitations: it is not ideal for amplifying small fragments of target nucleic acid, the primer designing is complex, usually four to six primers are required, and two primers of the set are usually long incurring extra cost in synthesis.
Nucleic acid sequence-based amplification (NASBA)
Nucleic acid sequence-based amplification (NASBA) was proposed by Compton in (1991), which is very similar to transcription-mediated replication (TMA) and self-sustained sequence replication (3SR). The enzymatic reaction for amplification requires three enzymes, avian myeloblastosis reverse transcriptase, T7 RNA polymerase and RNase H, and two primers: P1 and P2. P1 carries a binding site for T7 DNA polymerase and helps in the synthesis of RNA from the RNA–DNA hybrid with the help of reverse transcriptase. Once the RNA is extracted from the hybrid, it is degraded by RNAse H; the rest of the cDNA is accessible to P2, which helps synthesize complementary strands (Fakruddin et al. 2012). Due to the involvement of reverse transcriptase, this technique is better than any other for RNA amplification. NASBA can be performed to achieve RNA amplification up to 109-fold copies in approximately 90 min at 41 °C without using a thermocycler. RNA detection using NASBA can be achieved by using different types of techniques such as the dideoxy method which includes reverse transcriptase and a labeled oligonucleotide primer, enzyme-linked gel assay (ELGA), electrochemiluminescent detection, molecular beacon detection, southern hybridization and fluorescent correlation spectroscopy (Compton 1991). Kievits et al. (1991) described the use of NASBA in the detection of HIV-1; since then, it has been improved considerably and became commercially available as NASBA HIV-1 QT by Biomerieux Ltd.
Gabrielle et al. (1993) combined enzyme-linked gel assay (ELGA) with NASBA for rapid species-specific identification of mycobacterium in clinical samples. They designed specific probes according to the conserved region of 16 s rRNA of different mycobacterium species with other similar bacteria. The technique was able to identify Mycobacterium tuberculosis correctly in less than 6 h by the formation of Mycobacterium tuberculosis complex single probe. Loeffeler et al. (2001) used NASBA to detect Aspergillus species, which is the main causative agent of aspergillosis in humans. He compared the NASBA technique with previously published RT-PCR technique by sequencing of 18 s rRNA from five types of Aspergillus species. The NASBA technique was able to identify the Aspergillus species in 6 h which was less than that using the RT-PCR technique. Sugiyama et al. (2003) combined southern hybridization with NASBA for the identification of the rabies virus. They also compared the test result with a parallel-running RT-PCR technique combined with southern hybridization. Although it took 8 h for the complete detection of the DNA yet, it proved to be a better option for specific RNA detection than RT-PCR. Saeedinia et al. (2008) identified coxsackievirus B3 in virus-infected cell culture through RT-PCR and NASBA techniques. They performed the test on a cell line and specimen organs of artificially infected mice. They evaluated that both the techniques can be used for detection of CVB3, but NASBA is simpler to perform in comparison to RT-PCR.
G-quadruplex DNAzyme assay is an emerging technique in molecular diagnostics and its integration with NASBA has proved useful for visual detection of DNA. Lu et al. (2017) developed a novel method by integrating G quadruplex DNAzyme assay with NASBA to detect different strains of classical swine flu virus (CSFV). The reaction was carried out for approximately 3 h, after which the assay detected ten copies/ml of CSF viral RNA. RNA Mango technology is also one of the very useful techniques in detecting RNA by using a thiazole orange bifunctional dye. This fluorogenic method was used for detection through NASBA by nesting the primers and modifying the inner primers so that they can encode RNA Mango aptamer sequence. This technique detected ~ 25 pM (1.5 × 107 RNA/μL) of E. coli ClpB RNA template (Abdolahzadeh et al. 2019). NASBA has also been used to detect Apple scar skin viroid in apple cultivars. This technique uses a molecular beacon and provides a real-time quantification assay to certify disease-free apple cultivars. Results were compared with RT-PCR and it was found that RT-NASBA can detect 1 × 105 copies and was more sensitive (Heo et al. 2019).
NASBA has also been integrated into miniaturized lab-on-a-chip systems, which can help in detection mechanisms in novel pathogen capture technology. Its sensitivity to laboratory-based NASBA system has been described in many previous publications in both nano and microliter volumes with molecular beacon detection using centrifugal microfluidic devices. In conclusion, NASBA has the advantage of directly amplifying target RNA. It generates a high copy number at constant temperature and is commercially available as a miniature device. However, it has a few limitations: it is less efficient in amplifying longer RNA targets, requires a denaturation step to initiate the process and requires multiple enzymes, which make the process costly.
Strand displacement amplification (SDA)
Strand displacement amplification (SDA) is truly an isothermal amplification technique and does not require DNA denaturation for initiation, as it is carried out by strand displacement activity with the help of DNA polymerase and restriction enzyme to produce copies of DNA (Walker et al. 1992a, b). In the same year, Walker and his co-workers improved the early design of the SDA technique with the target generation step, which was not present in the earlier step. In the previous design, target molecules were amplified instead of their use in the target generation step. Nearly 107-fold amplification is achieved in 2 h at 37 °C. The three main important parts of this technique are repeated nicking of target sequences with the help of restriction enzymes, which are copied through a target generation process, followed by exponential amplification of those target sequences. The target DNA sample is heat denatured in the presence of an excess of four primers. Two primers, SI and S2, are important SDA primers, which have target-binding regions at their 3'-ends and HincII recognition sequences (5'GTTGAC) located at the 5'-ends to the target sequences. The other two primers (B1 and B2) consist simply of target-binding regions (Walker et al. 1992a, b).
The SDA technique was later coupled with many types of detection techniques for rapid identification of pathogens in biological samples. Spargo et al. (1993) combined SDA with chemiluminescent detection method in which they used non-isotopic microtiter plate assay employed with biotinylated oligodeoxynucleotide probe and an alkaline phosphatase-conjugated oligodeoxynucleotide probe. They amplified target DNA in IS6110 insertion element of species within the Mycobacterium complex, which included five types of species with different target nucleic acid sequence copies. Walker et al. (1996), described the use of the fluorescence polarization (FP) technique with SDA. According to them the previous studies that include the use of the padlock probe and chemiluminescent assay were not suitable to be performed in a closed system, as they require separation of strands, whereas the FP opens the door to use SDA in a closed system thus terminating the initial heat denaturation step. Later on, Hellyer et al. (1999) used reverse transcriptase enzyme with SDA technique to detect mRNA in the Mycobacterium tuberculosis complex. According to them, the presence of mRNA is a good indicator of bacterial viability compared to DNA and rRNA for therapeutic diagnosis.
Shi et al. (2014) described the use of miRNAs for the detection of pathogens using the exponential SDA technique. They used Klenow fragment, nicking enzyme Nt.AlwI and two primers, and the miRNA target can trigger two cycles of nicking, polymerization and displacement reactions. These reaction cycles amplified the target miRNA exponentially and generated dsDNAs detectable with SYBR Green I in real-time PCR. Du et al. (2016) integrated the SDA technique with G-quadruplex DNAzyme-based sensing system and tested the activity of uracil-DNA glycosylase to evaluate the sensitivity of the technique. They used DNA templates with different nicking sites and compared them to conclude an optimal nicking site number which is increased from one to two sites. This enhanced the SDA technique as an exponential amplification technique for the development of a sensor with a sensitivity range of 1 × 10–4-1 U/mL. The use of three-way junction probes is a concept of nucleic acid hybridization for DNA target recognition and signal amplification. Wang et al. 2017 used this concept in which G-rich single sequence is formed by a downstream strand displacement amplification. This sequence has similar properties to peroxidase, which can be quantified using H2O2 and TMB (Figs. 5, 6, 7, 8).
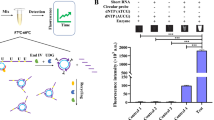
Methyltransferase (MTase)-assisted SDA technique for detection of MTase activity. Molecular beacons labeled with FAM and TAMRA as fluorophore for visual detection. Reprinted with permission from Cui et al. (2019).
Working of fluorescent biosensor based on HDA for detection of β-glucosyltransferase. A β-GT catalyzes the transfer of β-glucosyl residue from UDP-glucose to form 5-ghmc. B Schematic diagram of fluorescent biosensor based on SYBR Green for detection of β-GT (reprinted with permission from Liu et al. (2020a, Copyright
Detection of β-estradiol (E2) using electrochemical biosensor based on EXPAR and HCR ssDNA is produced through EXPAR. HCR signal output detects ssDNA using the turn off–turn on mechanism at 520 nm. Avidin-modified MNPs attached with biotin modified the E2-aptamer for detection of β-estradiol (E2) (reprinted with permission from Wang et al. (2020a, b).
Schematic diagram of SPIA. SPIA has a blocker, RNAse H and DNA polymerase. A, B DNA/RNA primer is first amplified at constant temperature where (C, D) RNAse H dissolves the RNA and extension continues. E, F A new DNA/RNA primer binds on the exposing part and extension continues. G Blocker stop the ssDNA amplification once the extension proceed to the blocker subsequently producing large number of ssDNA
A fluorescent method for the detection of virus DNA is developed by coupling the carbon nanodots (CDs) with isothermal strand displacement amplification (iSDA) technology. This technique proved to be ultrasensitive and specific for viral DNA detection with an LOD of 4.6 × 10−15 for H7 gene and 3.4 × 10−15 m for N9 gene, respectively. Hence, this technique proved to be a better option for viral DNA detection in biological samples and can contribute to wide applications in the field of clinical diagnostics (Yang et al. 2019). MTase-assisted SDA is used to develop a DNA-based biosensor that can detect MTase activity with a one-step method of achieving LOD of 3.3X10−6 U mL−1. Molecular beacons used in this technique are labeled with FAM and TAMRA fluorophores at both ends (Cui et al. 2019). A simple and label-free method was developed for the detection of S1 nuclease activity by designing a DNA probe which will serve as a substrate to carry out the amplification. They showed that in the presence of the S1 nuclease enzyme, the DNA probe is cleaved, resulting in reduced fluorescence of nucleic acid-specific dye SYBR Green I, while in the absence of S1 nuclease, fluorescence was maintained. This technique detected S1 nuclease activity down to 0.000087 U/μL (Lee et al. 2019a, b).
Wang et al. (2020a, b) developed an electrochemical biosensor based on NsbI restriction enzyme-mediated strand displacement amplification integrated with four-way DNA junction. This biosensor was used to detect PIK3CAH1047R gene mutation by inducing the increase in the number of DNA copies of target gene with the help of NsbI restriction enzyme. DNA junction coupled with methylene blue was able detect PIK3CAH1047R target gene with LOD as low as 0.001%.
The SDA is being used in miRNA detection, making it a useful tool for screening and diagnosing various disease conditions. However, the nuclease selection and sample preparation make it challenging.
Rolling circle amplification
Rolling circle amplification was reported in 1998, which can utilize circular templates to produce long linear strands with the help of DNA polymerases. The linear strand contains multiple copies of the target sequence. It can be associated with ligating a padlock probe to provide sensitive detection of nucleic acids (Baner et al. 1998). If compared to SDA, which requires nicking endonucleases (NEase), RCA only requires DNA polymerases to carry out the whole reaction, but an additional ligase is also required to use for the specific circularization of a padlock probe using the target sequence of DNA, which acts as a template in ligation reaction (Ali et al. 2014). Since the development of this technique, it has been improved and has been used by many researchers for amplification procedures.
Marciniak et al. (2008) used circularized oligonucleotide probes, because it could detect short DNA sequences using RCA coupled with LAMP to obtain better results. RCA and antibody-induced DNA strand displacement were integrated to generate repeated G-quadruplex DNAzymes in large quantities to develop a colorimetric technique to detect anti-HCV antibodies (Gao et al. 2019a, b). An RCA technique was developed for telomerase activity detection using the catalytic hairpin assembly (CAR) method, which was successfully implemented for highly sensitive in vitro and in- itu detection of telomerase activity (Liu et al. 2020a, b). Ravikumara et al. (2020) synthesized nucleotide dUrkTP which was a highly fluorescent naphthalimide deoxyuridine triphosphate that undergoes aggregation-induced emission (AIE). They used this fluorescent material in primer extension during amplification to detect microRNA 24-3P. The proposed method detected targets with high sensitivity and LOD of 3.58 fM. In another study, a label-free fluorescent assay was used to detect microRNA. Here in nicking enhanced RCA induced by G-Quadruplex formation with quenching of MoS2 quantum dots by inner filter effect was demonstrated. The proposed method was highly selective toward microRNA with LOD of 4.6 fM (Ge et al. 2020). PDMS microfluidic chip earned a great response in the field of diagnostics, but due to some limitations the need for a new microfluidic technique for RCA has been aroused. This need was fulfilled by Feng and his co-workers by developing an open space microfluidic chip for semi-quantitative detection of vascular endothelial growth factor (VEGF) using RCA (Feng et al. 2019).
In the past few years, viral diseases have emerged as a serious concern worldwide and, due to a lack of resources, on-site and fast diagnosis of these diseases is still an issue. Ciftci et al. (2020) developed a padlock probe (PLP)-based rolling circle amplification for the detection of the Ebola virus. They used two PLP-based assays for Ebola virus detection and demonstrated multiplexing capability for simultaneous detection of Ebola, Zika and dengue virus. They also developed a microfluidic RCP enrichment (MRE) chip based on capillary flow for digital quantification without need of any special instrument. A modification in RCA was done by Tian et al. (2020) by combining two padlock probes and RCA reaction rounds in a one-pot amplification reaction named circle to circle amplification (C2CA). The developed method was used to detect SARS-CoV2 with simultaneous real-time detection with amplification of nucleic acid. The developed method was able to achieve LOD of about three magnitudes lower than the LOD of the single round-based RCA assay.
Another variant of RCA called the ramification amplification method (RAM) is a modified version of RCA also known as hyperbranched RCA, cascade RCA or exponential RCA. RAM works with two primer amplification, unlike RCA, a single primer-initiated amplification. Yao et al. (2009) developed a sensitive and specific real-time assay for the detection of miRNAs. The developed method has a dynamic range of seven orders of magnitude for quantification ranging from 103 to 1010 copies per reaction. Beals et al. (2010) showed that electrophoresis of RAM products yields characteristic dsDNA ladder. Their experiment showed that equal fluorescence with increasing size of bands suggests a decrease in molecule number, which is proportional to the length of a DNA molecule. Ramified RCA reaction was used to produce milligrams amount of nucleosomal DNA. The experiment was performed by producing genomic DNA from human LIN28B locus and showed that it can form functional nucleosomes capable of binding transcription factor Oct4 (Emmerik et al. 2019). In PCR-based nucleic acid amplification, the amplified products are template DNA clones. However, the amplified product in RCA is a long repeating DNA. In RCA, an additional step is required to synthesize the circular DNA. These circular DNAs show unusual topological issues and hinder the activity of DNA polymerase; Phi29 DNA polymerase has been employed in RCA to address the topological issues as it has fantastic strand displacement abilities at isothermal conditions. The 3’exonuclease activity of Phi29 DNA polymerase enables the RCA to use mRNA as a template and PLP simultaneously. However, RCA faces challenges in specifically binding targets due to mispriming caused by nonspecific interaction. In RCA, the target binding is limited to a single interaction between the target and primer compared to primer binding at multiple locations in PCR, CPA and LAMP. The diverse application of RCA makes it a powerful tool for the sensitive detection of DNA, RNA, protein and other analytes including whole cell. In addition to detecting the above-mentioned targets, RCA has industrial use in the production of DNA aptamers and cell-free protein, rolling circle transcription, nanoribbons and DNA nano-scaffolds for synbodies-based method development. However, RCA has a few limitations such as the requirement of circular template, complex primer and linear amplification process resulting in low yield of the amplified product.
Other isothermal amplification techniques
Whole genome amplification
Whole genome amplification by isothermal methods was reported lately by Li et al. which is a primase-based DNA amplification system. The so-called whole genome amplification (WGA) method from Harvard University has shown promising results in case when total DNA needs to be amplified (e.g., DNA archiving, single cell analysis, tracing of DNA contaminations, or forensics). Three types of strategies are used for whole genome amplification: single cell comparative genomic hybridization (SCOMP), multiple displacement amplification (Repli-G) and a combination of displacement pre-amplification and PCR amplification (Picoplex and MALBAC) (Borgstrom et al. 2017). There are many preferred methods to perform this technique based on the target and primers used such as multiple strand displacement amplification and whole genome strand displacement amplification (Deleye et al. 2017). In the first one, two sets of primers are used, which are denoted as left set and right set, whereas in another method a random set of primers is used to amplify a genomic nucleic acid. Multiple displacement amplification technique requires multiple primers for strand displacement replication of nucleic acids (Lovmar and Syvanen 2006). Multiple cross displacement amplification is another method that includes a set of ten primers i.e., two cross primers (CP1, CP2), two displacement primers (F1, F2) and six amplification primers (D1, C1, R1, D2, C2, R2) along with a polymerase, which works complementarily to achieve a successful amplification of nucleic acid (Wang et al. 2015). The WGA is applicable in Y chromosome short tandem repeat analysis in forensic, SNP detection and haploid typing. However, there are few disadvantages in WGA: large amplification deviation in case of low abundance of template and poor miniaturization capabilities.
Cross priming amplification
Cross priming amplification (CPA) is another class of iNAAT’s reactions which utilizes multiple primers and probes, in which one or more is a cross primer. Unlike other methods that require additional proteins to nick the DNA to allow amplification by strand displacement, it does not need any additional protein (Xu et al. 2012). In the CPA assay, the 5ʹ end of the cross primer is not complementary to the template and will be displaced when DNA polymerase extends the upstream displacement primer using multiple cross-linked primers (six to eight primers), and a DNA target sequence can be amplified at a constant temperature. The detection of amplified products is performed on a lateral flow strip housed in an enclosed, sealed plastic device to prevent the leakage of amplicons (Zhang et al. 2012). Zhang et al. (2019a, b, c, d) labeled two primers of CPA with biotin and fluorescein isothiocyanate (FITC), which allows the amplified dual labeled product to be detected using disposable contamination-proof cartridge that contains a nucleic acid detection strip (NADS). This technique is used for the rapid detection of B. cereus, which is visible to the naked eye as colored lines with a sensitivity of 3.6 × 101 CFU/mL. CPA has also been coupled with test strips for the detection P. aeruginosa. Primers targeting the oprl gene of P. aeruginosa were designed for rapid detection, which resulted in LOD of 1.18 × 102 copies/μL for plasmid DNA and 4.4 CFU/mL for bacteria in pure culture, and were found to be more sensitive than the conventional PCR methods (Yong et al. 2020). Zheng et al. (2020) developed a technique for multiplex detection of different meats by coupling CPA with colloidal gold nucleic acid detection strips to differentiate between meat of cow, Arctic fox, sheep and pig. Meat of fox and pig was found to be feasible with a detection limit of 1% for fox meat. The CPA offers flexibility in primer designing and the flexibility of the ratio of primers in the reaction. The CPA has application in the determination of specific alleles and lateral flow stripes in the spot detection of viruses. The limitation of CPA is the long amplification time and difficulty detecting a trace number of targets.
Helicase-dependent amplification
In 2004, helicase-dependent amplification (HDA) was introduced, based upon the naturally occurring process of DNA replication. In this amplification process, the helicase enzyme helps unwind the target DNA strand at a temperature of 37 °C to carry out the heat-induced denaturation step of PCR (Vincent et al. 2004). The unwinding is stimulated by a protein known as MutL protein and a second protein known as single-stranded binding protein (SSB), which prevents the re-hybridization of the separated ssDNA targets. Primers and DNA polymerases hybridize to the ssDNA and the extension process is carried out. This exponential reaction can produce million-fold copies of target DNA in 60–120 min. An experiment was performed to determine fecal pollution in environmental waters with the help of HDA. Test strips were coupled with HDA to detect ruminant-associated genetic markers (16 s rRNA) in fecal members of phylum Bacteroides with the help of sandwich DNA hybridization reactions. Analytical LOD95% for HDA strip assay was 7.3 copies per reaction, which was better than qPCR assay: 3.7 copies per reaction (Kolm et al. 2019). A fluorescent biosensor was also developed for the detection of β-glucosyltransferase, in which the amplification of nucleic acid is triggered with the help of 5-hydroxymethylcytosine glucosylation. When exponential amplification is achieved, dsDNAs produced were detected using a specific dye SYBR Green 1 with LOD of 2.79 × 10–5 U/mL (Liu et al. 2020a, b). Unlike PCR, HDA uses helicase enzyme as opposed to temperature-dependent denaturation, leading to temperature-independent amplification of DNA. This offers a DNA amplification to occur in a point-of-need setting. However, the primer binding at constant temperature tends to be nonspecific due to the lack of stringent temperature requirements for primer binding, leading to nonspecific priming and false positive results.
Exponential amplification reaction
Exponential amplification reaction (EXPAR) is used for amplification of short oligonucleotides in addition to polymerase strand extension and single strand nicking (Shi et al. 2014). The reaction can yield 106- to 109-fold copies of target DNA under isothermal conditions within minutes. Consequently, it is a simple, low-cost, and highly sensitive method that can contribute in the future advancement in research on the biological roles of short oligonucleotide sequences in clinical diagnostics (Van et al. 2003). Jia and co-workers demonstrated that this method can be used for efficient amplification of short miRNAs. This assay results in a huge range of over ten orders of magnitude and high specificity to clearly discriminate even a one base difference in sequences. An improvement in EXPAR is introduced known as Nicking enzyme amplification reaction (NEAR), as it can allow any target amplification by inserting nicking enzyme recognition sites inside of the targeted gene sequence (Troger et al. 2015). EXPAR is used to develop a label-free ‘one-pot’ biosensor for ultrasensitive detection of polynucleotide kinase (PNK), which was also successfully used for screening of PNK inhibitors and PNK activity in a single cell (Zhang et al. 2019a, b, c, d). A sensitive fluorescent biosensor was developed for the detection of 17 β-estradiol (E2) which is present in environment and food and is responsible for endocrine disruption which affects the growth and development in organisms. Both the techniques, EXPAR and HCR (hybridization chain reaction), were used to amplify cDNA to multiple copies of long dsDNA, which were labeled with FAM (Carboxyflourescein) and BHQ-1 (Black hole quencher). This two-step method EXPAR showed a LOD of 0.37 pg/mL compared to the LOD of 34.33 pg/mL obtained from single HCR method. This method was also used to detect E2 in water and milk and is found suitable for the detection of actual samples (Wang et al. 2020a, b). Application of EXPAR includes detection of nucleic acids, protein, enzyme activity and metal ions. Additionally, EXPAR can amplify nucleic acid at room temperature with low tolerance to biological substances and inhibitors. However, it requires a nicking enzyme to facilitate the reaction.
Recombinase polymerase amplification (RPA)
Recombinase polymerase amplification (RPA) was first introduced by Nial Armes in 2006 which uses a recombinase protein (with ATP as an energy source) and SSB protein to match primers to the target on the template strand. A DNA polymerase isolated from Staphylococcus aureus, i.e., Sau polymerases, then extends the primer which then displaces the native strand. It can produce millions of copies in 40 min at 37°–42° C. One of the main advantages of RPA is that it can be conducted in a single tube and results are given in ‘real time’ (Piepenburg et al. 2006). It has been associated with FRET-based fluorescence probes and lateral flow strip-sensing systems for clearance of background noise and instrument-free analysis. Various RPA kits have also been commercialized by Twist Dx, Cambridge, UK (Craw and Balachandran 2012). A fluorescence assay using RPA was developed for the detection of Schistosoma haematobium by targeting Dra1 genomic repeat region, which showed a positive fluorescence result and found to be suitable for detection of S. haematobium (Rostron et al. 2019). A novel method for ultrasensitive detection of E. coli O157:H7 was developed using RPA-based LFD. LOD was found to be 4.4 × 10 CFU/mL, which was 100 times lower than that of PCR, resulting in higher sensitivity of RPA for detecting E. coli in food samples (Hu et al. 2020). RPA is a rapid method mainly applied to detect HIV, tuberculosis and food quality control. However, the high template concentration inhibits RPA, and the detection of amplified products is difficult using gel electrophoresis.
Signal-mediated amplification of RNA technology (SMART)
Signal-mediated amplification of RNA technology (SMART) is based on three-way junction (3WJ), which works on signal amplification rather than thermocycling and copying of target sequences (Hall et al. 2002). It can be used to detect specific target sequences of RNA or DNA. It consists of two single-stranded oligonucleotide probes. One is called an extension probe, and another is a template probe. These two probes, upon target binding, forms a 3WJ similar to a holiday junction. The longer template probe contains T7 RNA promoter sequence, followed by the transcription template overhang region. The transcription region generates multiple copies of RNA using RNA polymerase, which further serves as a target analyte. To enhance the sensitivity of SMART, in the near future it can be coupled with CRISPR-based SHERLOCK (specific high-sensitivity enzymatic reporter unlocking) technology (Gootenberg et al. 2018).
Besides genome editing tool, CRISPR has been adapted for RNA detection. The CRISPR-associated enzyme Cas13/Cas12 exhibits nonspecific endonuclease activity after binding to the target RNA. The collateral activity of the Cas endonuclease cleaves the reporter molecule to generate a signal. The crRNA can be designed, as it can discriminate the single nucleotide polymorphism among the variants. Coupling of SMART with CRISPR/Cas system will enhance the specificity and sensitivity of detection to at par level and may be useful in handling COVID-19 kind of situations. SMART is a highly sensitive method for detection of very low copy numbers of target analyte. The generated RNA molecule could be coupled with enzyme-linked oligosorbent assay (ELOSA), molecular beacon or the above-mentioned CRISPR-based detection. However, the linear amplification process comparatively requires more time to generate a detectable signal. The requirement of T7 promoter and polymerases makes it tricky and costly.
Single primer isothermal amplification
Single primer isothermal amplification (SPIA) technique is a novel rapid iNAAT method, which depends on a target-specific primer containing 3′-DNA sequence portion and 5′-RNA sequence portions (Kurn et al. 2005).
The SPIA comprises DNA polymerase with strong strand-displacement activity, RNase H and blocker (helps in stopping primer extension). Yang et al. (2018a, b, )c developed a method based on SPIA for detection of S. aureus in food by targeting accessory gene regulator (agr). Compared with conventional PCR, the developed method has 100-fold higher sensitivity with LOD of 4.3 × 100 CFU/mL. Alicyclobacillus acidoterrestris is a major spoilage causing pathogen in foods, and it has the ability to survive and multiply during the pasteurization process. A method based on SPIA for detection of this pathogen in apple juices was developed by (Yang et al. 2017) targeting 16 s rRNA gene with a DNA/RNA chimeric primer. The developed SPIA method was highly sensitive for specific pathogen with LOD of 4.8 CFU/mL. SYBR Green 1 dye is used with SPIA for rapid detection of Listeria monocytogens in raw chicken targeting hlyA gene. This real-time rapid method was able to show sensitivity of 1.4 × 101 CFU/mL by precipitate and 1.4 × 100 CFU/mL by fluorescence which was tenfold higher than conventional PCR methods (Yang et al. 2020). Similar to SMART, SPIA could be coupled with ELOSA, molecular beacons and CRISPR-based assays for enhanced sensitivity and specificity. However, there are a few limitations such as using a single primer, low temperature reaction and initial denaturation step.
Dual priming isothermal amplification (DAMP)
A novel technique was reported named dual priming isothermal amplification (DAMP), which has the advantage of dual priming strand extension strategy, due to which it is highly sensitive for rapid nucleic acid detection with very low nonspecific signals. It uses six primers: (i) two outer primers (FO and RO), (ii) two inner primers (FI and RI) and (iii) two pairing-competition primers (FC and RC). It is based on two steps: (i) basic structure-producing step and (ii) cycling amplification step. This assay was used for the detection of HIV-1 DNA/RNA and E. coli DNA with a slight increase in sensitivity in shorter duration as compared to LAMP and PCR. However, the technique can be more improvised in the future and can replace the currently used techniques (Ding et al. 2019). The DAMP assay is capable of detecting target analytes rapidly. More importantly, is shows ultralow signal background. However, a long list of primers and prior sequence information of the target nucleic acid are a few obligations.
Conclusion
NASBA, SDA, EXPAR, HDA, RPA and SPIA are complex protocols that require multiple enzymes (two or more), rigorous optimization and special reagents. Only a few assays (e.g., RCA, LAMP, and CPA) can be efficiently carried out at a fixed temperature using one enzyme. However, RCA was restricted to amplifying the circular target DNA, limiting the usefulness of the technique, whereas SDA needs an initial denaturation step and a longer processing time limiting its use in POC. Although LAMP and CPA methodologies have been verified to be useful for basic, clinical and applied research, the trace amounts of target sequences were still difficult to detect in various samples. LAMP has been found to be the most popular and preferred technique in recent years due to its simplicity and reliability. RT-LAMP is considered as a rapid and sensitive diagnostic technique for the recent pandemic of COVID-19 and other viral infections in patients. Although LAMP produces high noise background signals when used for multiple target sites, an improvement called DAMP can be used for short analytes to gain highly sensitive molecular detection with low nonspecific signals. The nonspecific amplification is obvious in isothermal amplification due to the stringency of primer binding being compromised. Several additives have been used to enhance the specificity of isothermal amplification methods, namely, polyethylene glycol, dextran, proline, betaine, pullulan and helicases. Pullulan has also been reported to increase the activity of CPA. The coupling of isothermal amplification techniques with methods such as colorimetric, nanoparticles, lateral flow biosensor etc., leads to the development of novel sensing technique. However, some techniques need to be optimized to produce better results and increase their use at POC site for diagnosis. Notably, each amplification technique has its specialty, which increases when coupled with other methods. Still, PCR has the advantage of nucleic acid cloning that IAT cannot perform.
Data Availability
Few images underlying in this study are not publicly available due to copyright restrictions. Such images are made available from the corresponding authors through reasonable request and proper permission.
References
Abdolahzadeh A, Dolgosheina EV, Unrau PJ (2019) RNA detection with high specificity and sensitivity using Nested Fluorogenic Mango NASBA. RNA 25(12):1806–1813
Abirami N, Nidaullah H, Chuah LO, Shamila-Syuhada AK, Chandraprasad SR, Huda N, Hasmaizal H, Rusul G (2016) Evaluation of commercial loop-mediated isothermal amplification-based kit and ready-to use plating system for detection of Salmonella in naturally contaminated poultry and their processing environment. Food Control 70:74–78
Akduman D, Ehret JMM, Messina K, Ragsdale S, Judson FNN (2002) Evaluation of a strand displacement amplification assay (BD ProbeTec-SDA) for the detection of Niesseria gonorrhoeae in urine specimens. J Clin Microbiol 40:281–283
Ali MM, Li F, Zhang Z, Zhang K, Kang DK, Ankrum JA (2014) Rolling circle amplification: a versatile tool for chemical biology, materials science and medicine. Chem Soc Rev 43:3324–3341
Aliotta JM, Pelletier JJ, Ware JL, Moran LS, Benner JS, Kong H (1996) Thermostable Bst DNA polymerase I lacks a 3′→5′ proofreading exonuclease activity. Genet Anal 12(5–6):185–195
Avelar DM, Carvalho DM, Rabello A (2019) Development and clinical evaluation of loop-mediated isothermal amplification (LAMP) assay for the diagnosis of human visceral leishmaniasis in Brazil. Hindawi BioMed Res Int 2019:8240784
Banér J, Nilsson M, Mendel-Hartvig M, Landegren U (1998) Signal amplification of padlock probes by rolling circle replication. Nucleic Acids Res 26:5073–5078
Beals TP, Smith HJ, Nietupski RM, Lane DJ (2010) A mechanism for ramified rolling circle amplification. BMC Mol Biol 11(94):1–10
Becherer L, Borst N, Bakheit M, Frischmann S, Zengerie R, Stelten FV (2020) Loop mediated isothermal amplification (LAMP)- review and classification of methods for sequence specific detection. Anal Methods 12(6):717–746
Borgstrom E, Paterlini M, Mold JE, Frisen J, Lundeberg J (2017) Comparison of whole genome amplification techniques for human single cell exome sequencing. PLoS ONE 12(2):e0171566
Capaul SE, Gorgievski-Hrisoho M (2005) Detection of enterovirus RNA in cerebrospinal fluid (CSF) using NucliSens EasyQ Enterovirus assay. J Clin Virol 32(3):236–240
Chen C, Zhao Q, Guo J, Li Y, Chen Q (2017) Identification of methicillin-resistant Staphylococcus aureus (MRSA) using simultaneous detection of mecA, nuc, and femB by loop-mediated isothermal amplification (LAMP). Curr Microbiol 74(8):965–971
Ciftci S, Neumann F, Abdurahman S, Appelberg KS, Mirazimi A, Nilsson M, Madaboosi N (2020) Digital rolling circle amplification-based detection of Ebola and other tropical viruses. J Mol Diagn 22(2):272–283
Compton J (1991) Nucleic acid sequence-based amplification. Nature 350:91–92
Connelly JT, Rolland JP, Whitesides GM (2015) “Paper machine” for molecular diagnostics. Anal Chem 87:7595
Craw P, Balachandran W (2012) Isothermal nucleic acid amplification technologies for point-of-care diagnostics: a critical review. Lab Chip 12:2469–2486
Cui YX, Feng XN, Wang YX, Pan HHY, Pan H, Kong DM (2019) An integrated-molecular-beacon based multiple exponential strand displacement amplification strategy for ultrasensitive detection of DNA methyltransferase activity. Chem Sci 10:2290–2297
Das D, Panigrahi PK (2020) CFD simulations for paper-based DNA amplification reaction (LAMP) of Mycobacterium tuberculosis—point-of-care diagnostic perspective. Med Biol Eng Compu 58(2):271–289
Deleye L, Tilleman L, Plaetsen AV, Cornelis S, Deforce D, Nieuwerburgh FV (2017) Performance of four modern whole genome amplification methods for copy number variant detection in single cells. Sci Rep 7:3422
Deng Y, Liu Y, Jiang Z, Wang J, Zhang Q, Qian Y, Yuan Y, Zhou X, Fan G, Li Y (2019) A multiplex loop-mediated isothermal amplification assay for rapid detection of Bacillus cereus and Staphylococcus aureus. Biosci Trends 13(6):510–515
Diego JG, Fernández-Soto P, Crego-Vicente B, Alonso-Castrillejo S, Febrer-Sendra B, Gómez-Sánchez A, Vicente B, López-Abán J, Muro A (2019) Progress in loop-mediated isothermal amplification assay for detection of Schistosoma mansoni DNA: towards a ready-to-use test. Sci Rep 9:14744
Ding X, Wu W, Zhu Q, Zhang T, Jin W, Mu Y (2015) Mixed dye- based label-free and sensitive dual fluorescence for the product detection of nucleic acid isothermal multiple-self-matching-initiated amplification. Anal Chem 87(20):6–14
Ding X, Xu Z, Yin K, Sfeir M, Liu C (2019) Dual-priming isothermal amplification (DAMP) for highly sensitive and specific molecular detection with ultralow nonspecific Signals. Anal Chem 91:12852–12858
Du Y, Jiang H, Huo Y, Han G, Kong D (2016) Optimization of strand displacement amplification-sensitized G-quadruplex DNAzyme-based sensing system and its application in activity detection of uracil-DNA glycosylase. Biosens Bioielectron 77:971–977
Duan Y, Ge C, Zhang X, Wang J, Zhou M (2013) A rapid detection method for the plant pathogen Sclerotinia sclerotium based on loop-mediated isothermal amplification (LAMP). Australas Plant Pathol 43(1):61–66
Emmerik CLV, Gachulincova I, Lobbia VR, Daniëls MA, Heus HA, Soufi A, Nellisen HT, van Ingen H (2019) Ramified rolling circle amplification for efficient and flexible synthesis of nucleosomal DNA sequences. BioRxiv 43:676528
Fakruddin MD, Mazumdar RM, Chowdhury A, Mannan KB (2012) Nucleic acid sequence based amplification (NASBA)-prospects and applications. Int J Life Sci Pharma Res 2(1):106–121
Fakruddin M, Mannan KS, Chowdhury A, Mazumdar RM, Hossain MN, Islam S (2013) Nucleic acid amplification: Alternative methods of polymerase chain reaction. J Pharm Bioall Sci 5:245–252
Fan Z, Sun Y, Lin JM (2022) Self-assembled inkjet printer for droplet digital loop-mediated isothermal amplification. Chemosensors 10(7):247
Fang X, Chen H, Yu S, Jiang X, Kong J (2011) Predicting viruses accurately by a multiplex microfluidic loop-mediated isothermal amplification chip. Anal Chem 83:690
Feng S, Mao S, Dou J, Li W, Li H, Lin J (2019) An open-space microfluidic chip with fluid walls for online detection of VEGF via rolling circle amplification. Chem Sci 10:8571–8576
Fowler VL, Armson B, Gonzales JL, Wise EL, Howson EAL, Mistiaen ZV, Fouch S, Maltby CJ, Grippon S, Munro S, Jones L, Holmes T, Tillyer C, Elwell J, Sowood A, Peyer OD, Dixon S, Hatcher T, Patrick H, Laxman S, Walsh C, Andreou M, Morant N, Clark D, Moore N, Houhghton R, Cortes NJ, Kidd SP (2020) A highly effective reverse-transcription loop-mediated isothermal amplification (RT-LAMP) assay for the rapid detection of SARS-CoV-2 infection. J Infect 82(1):117–125
Gabrielle ME, Vilet VD, Schukkink RAF, Gemen BV, Schepers P, Klatser PR (1993) Nucleic acid sequence based amplification (NASBA) for the identification of mycobacteria. J Gen Microbiol 139:2423–2429
Gansen AM, Herrick IK, Dimov LP, Lee DTC (2012) Digital LAMP in a sample self-digitization (SD) chip. Lab Chip 12:2247
Gao T, Chai W, Shi L, Shi H, Sheng A, Yang J, Li G (2019a) A new colorimetric assay method for the detection of anti-hepatitis C virus antibody with high sensitivity. Analyst 144(21):6365–6370
Gao X, Sun B, Guan Y (2019b) Pullulan reduces the non-specific amplification of loop-mediated isothermal amplification (LAMP). Anal Bioanal Chem 411:1211–1218
Gaydos CA, Schwebke J, Dombrowski J (2017) Clinical performance of the Solana(R) point-of-care trichomonas assay from clinician-collected vaginal swabs and urine specimens from symptomatic and asymptomatic women. Expert Rev Mol Diagn 17(3):303–306
Ge J, Hu Y, Deng R, Li Z, Zhang K, Shi M, Yang D, Cai R, Tan W (2020) Highly sensitive microRNA detection by coupling nicking-enhanced rolling circle amplification with MoS2 quantum dots. Anal Chem 92(19):13588–13594
Gill P, Ghaemi A (2008) Nucleic Acid isothermal amplification technologies—a review. Nucleosides, Nucleotides Nucleic Acids 27(3):224–243
Gong J, Zhuang L, Zhu C, Shi S, Zhang D, Zhang L, Wang C (2016) Loop-mediated isothermal amplification of the sefA gene for rapid detection of Salmonella Enteritidis and Salmonella Gallinarum in chickens. Foodborne Pathog Dis 13(4):177–181
Gong L, Tang F, Liu E, Liu X, Xu H, Wang Y, Song Y, Liang J (2021) Development of loop mediated isothermal amplification assay combined with a nanoparticle based lateral flow biosensor for rapid detection of plasmid mediated colistin resistance gene mcr-1. PLoS ONE 16(4):e0249582
Gootenberg JS, Abudayyeh OO, Kellner MJ, Joung J, Collins JJ, Zhang F (2018) Multiplexed and portable nucleic acid detection platform with Cas13, Cas12a, and Csm6. Science 360(6387):439–444
Habiburrahman M, Ariq H, Handayani RRD (2021) Combining LAMP and Au-Nanoprobe to detect INH-RIF resistance accurately in tuberculosis: an evidence-based review. J Infect Dev Ctries 15(11):1555–1568
Hall MJ, Wharam SD, Weston A, Cardy DL, Wilson WH (2002) Use of signal-mediated amplification of RNA technology (SMART) to detect marine cyanophage DNA. Biotechniques 32:604–611
Hara-Kudo Y, Yoshino M, Kojima T, Ikedo M (2005) Loop-mediated isothermal amplification for the rapid detection of Salmonella. FEMS Microbiol Lett 253:155–161
He Z, Su Y, Li S, Long P, Zhang P, Chen Z (2019) Development and evaluation of isothermal amplification methods for rapid detection of lethal amanita species. Front Microbiol 10:15–23
Hellyer TJ, DesJardin LE, Teixeira L, Perkins MD, Cave MD, Eisenach KD (1999) Detection of viable Mycobacterium tuberculosis by reverse transcriptase-strand displacement amplification of mRNA. J Clin Microbiol 37(3):518–523
Heo S, Kim HR, Lee HJ (2019) Development of a quantitative real time nucleic acid sequence based amplification (NASBA) assay for early detection of Apple Scar Skin Viroid. Plant Pathol J 35(2):164–171
Ho NR, Lim GS, Sundah NR, Lim D, Loh TP, Shao H (2018) Visual and modular detection of pathogen nucleic acids with enzyme–DNA molecular complexes. Nat Commun 9(1):1–11
Hu L, Ma LM, Zheng S, He X, Hammack TS, Brown EW, Zhang G (2018) Development of a novel loop-mediated isothermal amplification (LAMP) assay for the detection of Salmonella ser. Enteritidis from egg products. Food Control 88:190–197
Hu J, Wang Y, Su H, Ding H, Sun X, Gao H, Geng Y, Wang Z (2020) Rapid analysis of Escherichia coli O157: H7 using isothermal recombinase polymerase amplification combined with triple-labeled nucleotide probes. Mol Cell Probes 50:101501
Huang X, Lin X, Urmann K, Li L, Xie X, Jiang S, Hoffmann MR (2018) Smartphone based in gel Loop mediated isothermal amplification (gLAMP) system enables rapid coliphage MS2 Quantification in Environmental Water. Environ Sci Technol 52:6399
Ignatov KB, Barsova EV, Fradkov AF, Blagodatskikh KA, Kramarova TV, Kramarov VM (2014) A strong strand displacement activity of thermostable DNA polymerase markedly improves the results of DNA amplification. Biotechniques 57:81–87
Jang WS, Lim DH, Yoon J, Kim A, Lim M, Nam J (2021) Development of a multiplex Loop-Mediated Isothermal Amplification (LAMP) assay for on-site diagnosis of SARS CoV-2. PLoS ONE 16(3):e0248042
Kanchanaphum P (2018) Identification of human DNA by loop-mediated isothermal amplification (LAMP) technique combined with white ring precipitation of Cu(OH)2. Songklanakarin J Sci Technol 40(4):738–742
Kievits T, Gemen BV, Strijp DV, Schukkink R, Dircks M, Adriaanse H, Malek L, Sooknanan R, Lens P (1991) NASBA isothermal enzymatic in vitro nucleic acid amplification optimized for the diagnosis of HIV-1 infection. J Virol Methods 35:273–286
Kim JH, Oh SW (2019) Development of a filtration-based LAMP-LFA method as sensitive and rapid detection of E. coli O157:H7. J Food Sci Technol 56(5):2576–2583
Kim S, Kim JH, Kim S, Park JS, Cha BS, Lee ES, Han J, Shin J, Jang Y, Park KS (2022) Loop mediated isothermal based nucleic acid lateral flow assay for the specific and multiplex detection of genetic markers. Anal Chim Acta 1205:339781
Kolm C, Martzy R, Fuhrer M, Mach RL, Krska R, Baumgartner S, Farnleitner AH, Reischer GH (2019) Detection of a microbial source tracking marker by isothermal helicase-dependent amplification and a nucleic acid lateral-flow strip test. Sci Rep 9:393–342
Kurn N, Chen P, Heath JD, Kopfsill A, Stephens KM, Wang S (2005) Novel isothermal, linear nucleic acid amplification systems for highly multiplexed applications. Clin Chem 51:1973–1981
Kurosaki Y, Magassouba NF, Oloniniyi OK, Cherif MS, Sakabe S, Takada A, Yasuda J (2016) Development and evaluation of reverse transcription-loop-mediated isothermal amplification (RT-LAMP) assay coupled with a portable device for rapid diagnosis of Ebola virus disease in Guinea. PLoS Negl Trop Dis 10(2):e0004472
Ledlod S, Bunroddith K, Areekit S, Santiwatanakul S, Chansiri K (2019) Development of a duplex lateral flow dipstick test for the detection and differentiation of Listeria spp. and Listeria monocytogenes in meat products based on loop-mediated isothermal amplification. J Chromatogr B 1139:121834
Lee SH, Baek YH, Kim YH, Choi YK, Song MS, Ahn JY (2017) One-pot reverse transcriptional loop-mediated isothermal amplification (RT-LAMP) for detecting MERS-CoV. Front Microbiol 7:2166
Lee CY, Kim H, Kim HY, Park KS, Park HG (2019a) Fluorescent S1 nuclease assay utilizing exponential strand displacement amplification. Analyst. https://doi.org/10.1039/C9AN00300B
Lee JW, Nguyen VD, Seo TS (2019b) Paper-based molecular diagnostics for the amplification and detection of pathogenic bacteria from human whole blood and milk without a sample preparation step. BioChip J 13(3):243–250
Li Y, Kim HJ, Zheng C, Chow WH, Lim J (2008) Primase-based whole genome amplification. Nucleic Acids Res 36:e79
Lin X, Huang X, Urmann K, Xie X, Hoffmann MR (2019) Digital loop mediated isothermal amplification on a commercial membrane. ACS Sensors 4:242–249
Liu C, Geva E, Mauk M, Qiu X, Abrams WR, Malamud D, Curtis K, Owen SM, Bau HH (2011) An isothermal amplification reactor with an integrated isolation membrane for point-of-care detection of infectious diseases. Analyst 136:2069
Liu L, Xu Y, Zhong W, Li L, Li W, Xiao Q (2018) Comparison of three terminal detection methods based on loop mediated isothermal amplification (LAMP) assay for spring viremia of Carp Virus (SVCV). Turk J Fish Aquat Sci 19(9):805–816
Liu M, Li CC, Luo X, Ma F, Zhang CY (2020a) 5-Hydroxymethylcytosine glucosylation-triggered helicase-dependent amplification-based fluorescent biosensor for sensitive detection of β-glucosyltransferase with zero background signal. Anal Chem 92(24):16307–16313
Liu Y, Li S, Zhang L, Zhao Q, Li N, Wu X (2020b) Catalytic hairpin-assembly assisted rolling circle amplification for high-sensitive telomerase activity. ACS Omega 5(20):11836–11841
Loeffler J, Hebart H, Cox P, Flues N, Schumacher U, Einsele H (2001) Nucleic acid sequence-based amplification of Aspergillus RNA in blood samples. J Clin Microbiol 39(4):1626–1629
Lovmar L, Syvanen A (2006) Multiple displacement amplification to create a long-lasting source of DNA for genetic studies. Hum Mutat 27(7):603–614
Lu X, Shi X, Wu G, Wu T, Qin R, Wang Y (2017) Visual detection and differentiation of Classis Swine Fever Virus strains using nucleic acid sequence based amplification (NASBA) and G-quadruplex DNAzyme assay. Sci Rep 7:44211
Marciniak JY, Kummel AC, Esener SC, Heller MJ, Messmer BT (2008) Coupled rolling circle amplification loop-mediated amplification for rapid detection of short DNA sequences. Biotechniques 45:275–280
Martzy R, Kolm C, Krska R, Mach RL, Farnleitner AH, Reischer GH (2019) Challenges and perspectives in the application of isothermal DNA amplification methods for food and water analysis. Anal Bioanal Chem 411:1695–1702
Mashooq M, Kumar D, Niranjan AK, Agarwal RK, Rathore R (2016) Development and evaluation of probe based real time loop mediated isothermal amplification for Salmonella: a new tool for DNA quantification. J Microbiol Methods 126:24–29
Mei X, Zhai X, Lei C, Ye X, Kang Z, Wu X, Xiang R, Wang Y, Wang H (2019) Development and application of a visual loop-mediated isothermal amplification combined with lateral flow dipstick (LAMP-LFD) method for rapid detection of Salmonella strains in food samples. Food Control 104:9–19
Mendes GM, Oliviera KG, Borba JC, Oliviera TS, Fiaccadori FS, Nogueira ML, Bailao AM, Soares CMA, Carrilho E, Duarte GRM (2019) Molecular diagnostics of dengue by reverse transcription-loop mediated isothermal amplification (RT-LAMP) in disposable polyester-toner microdevices. J Braz Chem Soc 30(9):1841–1849
Mohon AN, Getie S, Jahan N, Alam MS, Pillai DR (2019) Ultrasensitive loop mediated isothermal amplification (US-LAMP) to detect malaria for elimination. Malar J 18:350
Mori Y, Kitao M, Tomita N, Notomi T (2004) Real-time turbidimetry of LAMP reaction for quantifying template DNA. J Biochem Biophys Methods 59(2):145–157
Nagamine K, Hase T, Notomi TJMCP (2002) Accelerated reaction by loop-mediated isothermal amplification using loop primers. Mol Cell Probes 16(3):223–229
Nguyen VAT, Nguyen HV, Dinh TV et al (2018) Evaluation of Loopamp™MTBC detection kit for diagnosis of pulmonary tuberculosis at a peripheral laboratory in a high burden setting. Diagn Microbiol Infect Dis 90(3):190–195
Nguyen DV, Nguyen VH, Tae SS (2019) Quantification of colorimetric loop-mediated isothermal amplification process. BioChip J 13(2):158–164
Niemz A, Ferguson TM, Boyle DS (2011) Point-of-care nucleic acid testing for infectious diseases. Trends Biotechnol 29:240–250
Noren T, Unemo M, Magnusson C, Eiserman M, Matussek A (2014) Evaluation of the rapid loop-mediated isothermal amplification assay illumigene for diagnosis of Clostridium difficile in an outbreak situation. APMIS 122(2):155–160
Notomi T, Okayama H, Masubuchi H, Yonekawa T, Katanabe K, Amino N, Hase T (2000) Loop-mediated isothermal amplification of DNA. Nucleic Acids Res 28:E63
Notomi T, Mori Y, Tomita N, Kanda H (2015) Loop-mediated isothermal amplification (LAMP): principle, features, and future prospects. J Microbiol 53:1–5
Nzelu CO, Kato H, Peters NC (2019) Loop-mediated isothermal amplification (LAMP): an advanced molecular point-of-care technique for the detection of Leishmania infection. PLoS Negl Trop Dis 13(11):e0007698
Oh SJ, Park BH, Jung JH, Choi G, Lee DC, Kim DH, Seo TS (2016) Centrifugal loop-mediated isothermal amplification microdevice for rapid, multiplex and colorimetric foodborne pathogen detection. Biosens Bioelectron 75:293–300
Ohtsuka K, Yanagawa K, Takatori K, Hara-Kudo Y (2005) Detection of Salmonella enterica in naturally contaminated liquid eggs by loop-mediated isothermal amplification, and characterization of Salmonella isolates. Appl Environ Microbiol 71:6730–6735
Ordonez N, Salacinas M, Mendes O, Seidi MF, Meijer HJG, Schoen SD, Kema GHJ (2019) A loop-mediated isothermal amplification (LAMP) assay based on unique markers derived from genotyping by sequencing data for rapid in planta diagnosis of Panama disease caused by Tropical Race 4 in banana. Plant Pathol 68:1682–1693
Oriero EC, Jacobs J, Geertruyden JV, Nwakanma D, Alessandro U (2015) Molecular-based isothermal tests for field diagnosis of malaria and their potential contribution to malaria elimination. J Antimicrob Chemother 70:2–13
Panek J, Frąc M (2019) Loop-mediated isothermal amplification (LAMP) approach for detection of heat-resistant Talaromyces flavus species. Sci Rep 9:5846
Pang B, Fu K, Liu Y, Ding X, Hu J, Wu W, Xu K, Song X, Wang J, Mu Y, Zhao C, Li C (2018) Development of a self-priming PDMS/paper hybrid microfluidic chip using mixed-dye-loaded loop-mediated isothermal amplification assay for multiplex foodborne pathogens detection. Anal Chim Acta 1040:81–89
Park GS, Ku K, Baek SH, Kim SJ, Kim SI, Kim BT, Maeng JS (2020) Development of reverse transcription loop mediated isothermal amplification assays targeting severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). J Mol Diagn 22(6):729–735
Park I, Lim J, You S, Hwang MT, Kwon J, Koprowski K, Kim S, Heredia J, Stewart de Ramirez SA, Valera E, Bashir R (2021) Detection of SARS-CoV-2 Virus amplification using a crumpled graphene field-effect transistor biosensor. ACS Sens 6(12):4461–4470
Piepenburg O, Williams CH, Stemple DL, Armes NA (2006) DNA detection using recombination proteins. PLoS Biol 4:e204
Prasad D, Shankaracharya., Vidyarthi, A.S. (2011) Gold nanoparticles-based colorimetric assay for rapid detection of Salmonella species in food samples. World J Microbiol Biotechnol 27:2227–2230
Priya GB, Agarwal RK, Milton AAP, Mishra M, Mendiratta SK, Agarwal RK, Luke A, Singh BR, Kumar D (2018) Development and evaluation of isothermal amplification assay for the rapid and sensitive detection of Clostridium perfringens from chevon. Anaerobe 54:178–187
Priya GB, Agarwal RK, Milton AAP, Mishra M, Mendiratta SK, Agarwal RK, Luke A, Inbaraj S, Singh BR, Kumar D, Kumar GR, Rajkhowa S (2020) Rapid and visual detection of Salmonella in meat using invasin A (invA) gene-based loop mediated isothermal amplification assay. LWT-Food Sci Technol 126:109262
Quyen TL, Ngo TA, Bang DD, Madsen M, Wolff A (2019) Classification of multiple DNA dyes based on inhibition effects on real-time loop-mediated isothermal amplification (LAMP): prospect for point of care setting. Front Microbiol 10:2234
Rajalakshmi S (2017) Different types of PCR techniques and its applications. IJPCBS 7(3):285–292
Ravikumara GS, Pandith A, Seo YJ (2020) Highly fluorescent morpholine napthalimide deoxyuridine nucleotide for the detection of miRNA 24–3P through rolling circle amplification. Analyst 145:4777–4781
Reboud J, Xu G, Garrett A, Adriko M, Yang Z, Tukahebwa EM, Rowell C, Cooper JM (2019) Paper-based microfluidics for DNA diagnostics of malaria in low resource undeserved rural communities. PNAS 116(11):4834–4842
Rohatensky M, Livingstone D, Mintchev P, Barnes H, Nakoneshny S, Demetrick D, Dort J, Marle G (2018) Assessing the performance of a Loop Mediated Isothermal Amplification (LAMP) assay for the detection and subtyping of high-risk suptypes of Human Papilloma Virus (HPV) for Oropharyngeal Squamous Cell Carcinoma (OPSCC) without DNA purification. BMC Cancer 18(1):1–10
Rostron P, Pennace T, Bakar F, Rollinson D, Knopp S, Allan F, Kabole F, Ali SM, Ame SM, Webster BL (2019) Development of a recombinase polymerase amplification (RPA) fluorescence assay for the detection of Schistosoma haematobium. Parasites Vectors 12:514–521
Saad AA, Ahmed NG, Osman OS, Al-Basheer AA, Hamad A, Deborggraeve S, El-Safi S (2010) Diagnostic accuracy of the leishmania OligoC-TesT and NASBA-oligochromatography for diagnosis of leishmaniasis in Sudan. PLoS Negl Trop Dis 4(8):e776
Saeedinia A, Shamsara M, Zeinoddini M, Sadeghi V, Maghsoudi N (2008) Evaluation of nucleic acid sequence-based amplification (NASBA) and reverse transcription polymerase chain reaction for detection of coxsackievirus B3 in cell culture and animal tissue samples. Iran J Biotechnol 6(4):222–228
Saetiew C, Limpaiboon T, Jearanaikoon P, Daduang S, Pientong C, Kerdsin A, Daduang J (2011) Rapid detection of the most common high-risk human papillomaviruses by loop-mediated isothermal amplification. J Virol Methods 178:22–30
Salinas NR, Little DP (2012) Electric LAMP: virtual loop-mediated isothermal amplification. ISRN Bioinform 2012:696758
Sayad A, Ibrahim F, Mukim US, Cho J, Madou M, Thong KL (2018) A microdevice for rapid, monoplex and colorimetric detection of foodborne pathogens using a centrifugal microfluidic platform. Biosens Bioelectron 100:96–104
Schneider L, Blakely H, Tripathi A (2019) Mathematical model to reduce loop mediated isothermal amplification (LAMP) false-positive diagnosis. Electrophoresis. https://doi.org/10.1002/elps.201900167
Seyrig G, Stedtfeld RD, Tourlousse DM, Farhan A, Keara T, Cupples AM, Tiedje JM, Hashsham SA (2015) Selection of fluorescent DNA dyes for real-time LAMP with portable and simple optics. J Microbiol Methods 119:223–227
Shang Y, Sun J, Ye Y, Zhang J, Zhang Y, Sun X (2018) Loop-mediated isothermal amplification-based microfluidic chip for pathogen detection. Crit Rev Food Sci Nutr 60(2):201–224
Shi C, Liu Q, Ma C, Zhong W (2014) Exponential strand displacement amplification for detection of MicroRNAs. Anal Chem 86:336–339
Spargo CA, Haaland PD, Jurgensen SR, Shank DD, Walker GT (1993) Chemiluminescent detection of strand displacement amplified DNA from species comprising the Mycobacterium tuberculosis complex. Mol Cell Probes 7:395–404
Sudarsan S, Prabhu A, Prasad D, Mani NK (2023) DNA compaction enhances sensitivity of fluorescence-based nucleic acid assays: game changer in point of care sensors? Analyst. https://doi.org/10.1039/D3AN00102D
Sugiyama M, Ito N, Minamoto N (2003) Isothermal amplification of Rabies virus gene. J Vet Med Sci 65(10):1063–1068
Sun W, Qin P, Gao H, Li G, Jiao K (2010) Electrochemical DNA biosensor based on chitosan/nano-V2O5/MWCNTs composite film modified carbon ionic liquid electrode and its application to the LAMP product of Yersinia enterocolitica gene sequence. Biosens Bioelectron 25(6):1264–1270
Takano C, Seki M, Kim DW, Gardner H, McLaughlin RE, Kilgore PE, Kumasaka K, Hayakawa S (2019) Development of a novel loop-mediated isothermal amplification method to detect guiana extended-spectrum (GES) β-lactamase genes in Pseudomonas aeruginosa. Front Microniology 10:25
Texeira A, Paris JL, Roumani F, Dieguez L, Prado M, Espina B, Cela SA, Maestu GA, Lorenzo LR (2020) Multifunctional gold nanoparticles for the SERS detection of pathogens combined with a LAMP-in-microdroplets approach. Materials 13:1934
Tian B, Gao F, Fock J, Dufva M, Hansen MF (2020) Homogeneous circle to-circle amplification for real-time optomagnetic detection of SARS-CoV-2 RdRp coding sequence. Biosens Bioelectron 165:112356
Tong Y, Lemieux B, Kong H (2011) Multiple strategies to improve sensitivity, speed and robustness of isothermal nucleic acid amplification for rapid pathogen detection. BMC Biotechnol 11:50
Tran DH, Cuong HC, Tran HT, Le UP, Do HDK, Bui LM, Hai ND, Linh HT, Thao NTT, Anh NH, Hieu NT, Thang CM, Vu VV, Phung HTT (2020) A comparative study of isothermal nucleic acid amplification methods for SARS-CoV-2 detection at point of care. Biorxiv 37:113423
Troger V, Niemann K, Gartig C, Kuhlmeier D (2015) Isothermal amplification and quantification of nucleic acids and its use in microsystems. Nanomed Nanotech 6(3):1–19
Van NJ, Van LK, Galas DJ (2003) Isothermal reactions for the amplification of oligonucleotides. Proc Natl Acad Sci USA 100:4504–4509
Velders AH, Schoen C, Saggiomo V (2018) Loop-mediated isothermal amplification (LAMP) shield for Arduino DNA detection. BMC Res Notes 11:93
Vincent M, Xu Y, Kong H (2004) Helicase-dependent isothermal DNA amplification. EMBO Rep 5:795–800
Walker GT, Fraiser MS, Schram JL, Little MC, Nadeu JG, Malinowski DP (1992a) Strand displacement amplification- an isothermal, in vitro DNA amplification technique. Nucleic Acids Res 20(7):1691–1696
Walker GT, Little MC, Nadeu JG, Shank DD (1992b) Isothermal in vitro amplification of DNA by a restriction enzyme/DNA polymerase system. Proc Natl Acad Sci 89:392–396
Walker GT, Nadeu JG, Linn PC, Devlin RF, Dandliker WB (1996) Strand displacement amplification (SDA) and transient-state fluorescence polarization detection of Mycobacterium tuberculosis DNA. Clin Chem 42(1):9–13
Wang CH, Lien KY, Wang TY, Chen TY, Lee GB (2011) An integrated microfluidic loop-mediated-isothermal-amplification system for rapid sample pre-treatment and detection of viruses. Biosens Bioelectron 26(5):2045–2052
Wang Y, Wang Y, Ma A, Li D, Luo L, Liu D, Jin D, Liu K, Ye C (2015) Rapid and sensitive detection of nucleic-acid sequence by multiple cross displacement amplification. Sci Rep 5:11902
Wang X, Liu W, Yin B, Sang Y, Liu Z, Dai Y, Duan X, Zhang G, Ding S, Tao Z (2017) An isothermal strand displacement amplification strategy for nucleic acids using junction forming probes and colorimetric detection. Microchim Acta 184(6):1603–1610
Wang L, Chen G, Zhang C, Wang Y, Sun R (2019a) Application of loop mediated isothermal amplification with lateral flow dipstick to rapid and sensitive detection of Alexandrium catenella. Environ Sci Pollut Res 27(4):4246–4257
Wang Y, Wang Y, Jiao W, Li J, Quan S, Sun L, Wang Y, Qi X, Wang X, Shen A (2019b) Development of loop-mediated isothermal amplification coupled with nanoparticle-based lateral flow biosensor assay for Mycoplasma pneumoniae detection. AMB Expr 9:196
Wang T, Peng Q, Guo B, Zhang D, Zhao M, Que H, Wu H, Yan Y (2020a) An integrated electrochemical biosensor based on target-triggered strand displacement amplification and “four-way” DNA junction towards ultrasensitive detection of PIK3CA gene mutation. Biosens Bioelectron 150:111954
Wang Y, Zhao X, Zhang M, Sun X, Bai J, Peng Y, Li S, Han D, Ren S, Wang J, Han T, Gao Y, Ning B, Gao Z (2020b) A fluorescent amplification strategy for high-sensitive detection of 17 β-estradiol based on EXPAR and HCR. Anal Chim Acta 1116:1–8
Xu G, Hu L, Zhong H, Wang H, Yusa S, Weiss TC, Romaniuk PJ, Pickerill S, You Q (2012) Cross priming amplification: mechanism and optimization for isotehrmal DNA amplification. Sci Rep 2:246
Xu G, Nolder D, Reboud J, Oguike MC, Schalwyk DAV, Sutherland CJ, Cooper JM (2016) Paper-origami-based multiplexed malaria diagnostics from whole blood. Angew Chem Int Ed Engl 55:15250–15253
Xue Y, Luo X, Pang X, Zhou J, Wang J (2021) Optimizing the performance of digital loop-mediated isothermal amplification. Anal Biochem 631:114371
Yamamoto M, Alshahni MM, Tamura T, Satoh K, Iguchi S, Kikuchi K, Mimaki M, Makimurab K (2018) Rapid Detection of Candida auris Based on Loop-Mediated Isothermal Amplification (LAMP). J Clin Microbiol 56:e00591–18
Yan L, Zhou J, Zheng Y, Gamson AS, Roembke BT, Nakayama S, Sintim HO (2014) Isothermal amplified detection of DNA and RNA. Mol BioSyst 10:970–1003
Yan M, Li W, Zhou Z, Peng H, Luo Z, Xu L (2017) Direct detection of various pathogens by loop-mediated isothermal amplification assays on bacterial culture and bacterial colony. Microb Pathog 102:1–7
Yang Y, Yang Q, Ma X, Zhang Y, Zhang X, Zhang W (2017) A novel developed method based on single primer isothermal amplification for rapid detection of Alicyclobacillus acidoterrestris in apple juice. Food Control 75:187–195
Yang Q, Domesle KJ, Ge B (2018a) Loop-mediated isothermal amplification for Salmonella detection in food and feed: current applications and future directions. Foodborne Pathog Dis 15(6):309–331
Yang Q, Zhang Y, Li C, Zhao Y, Ma X, Zhang W, Zhang S (2018b) A rapid and visual single primer isothermal amplification-based method for detection of Staphylococcus aureus in raw pork products. Food Anal Methods 11:3113–3120
Yang X, Al-Attala MN, Zhang Y, Zhang A, Zang H, Gu C, Gao T, Chen Y (2018c) Rapid detection of Ustilaginoidea virens from rice using loop-mediated isothermal amplification assay. Plant Dis 102:1741–1747
Yang D, Guo Z, Wang J, Jin Y, Mei Q, Miao P (2019) Carbon nanodot-based fluorescent method for virus DNA analysis with isothermal strand displacement amplification. Part Part Syst Charact 36(10):1900273
Yang Q, Xu H, Zhang Y, Liu Y, Lu X, Feng X, Tan J, Zhang S, Zhang W (2020) Single primer isothermal amplification coupled with SYBR Green II: real-time and rapid visual methods for detection of Listeria monocytogenes in raw chicken. LWT Food Sci Technol 128:109453
Yao B, Li J, Huang H, Sun C, Wang Z, Fan Y, Chang Q, Li S, Xi J (2009) Quantitative analysis of zeptomole microRNAs based on isothermal ramification amplification. RNA 15(9):1787–1794
Yao X, Li P, Xu J, Zhang M, Ren R, Liu G, Yang X (2016) Rapid and sensitive detection of Didymella bryoniae by visual loop-mediated isothermal amplification assay. Front Microbiol 7:1372
Yin HY, Fang TJ, Wen HW (2016) Combined multiplex loop-mediated isothermal amplification with lateral flow assay to detect sea and seb genes of enterotoxic Staphylococcus aureus. Lett Appl Microbiol 63:16–24
Yong X, Ling Y, Cui X-CZ, Zhen LL, Peng L, Wei-Shang C (2020) Rapid detection of Pseudomonas aeruginosa by cross priming amplification. J Integr Agric 19(10):2523–2529
Yu L, Wu S, Hao X, Li X, Liu X, Ye S, Han H, Dong X, Li X, Li J, Liu N, Liu J, Zhang W, Pelechano V, Chen WH, Yin X (2020) Rapid colorimetric detection of COVID-19 coronavirus using a reverse transcriptional loop-mediated isothermal amplification (RT-LAMP) diagnostic platform: iLACO. medRxiv 9:20025874
Yuan D, Kong J, Li X, Fang X, Chen Q (2018) Colorimetric LAMP microfluidic chip for detecting three allergens: peanut, sesame and soybean. Sci Rep 8:8682
Yuan H, Chao Y, Shum HC (2020) Droplet and microchamber based digital loop mediated isothermal amplification (dLAMP). Small 16:1904469
Zanoli LM, Spoto G (2013) Isothermal amplification methods for the detection of nucleic acids in microfluidic devices. Biosensors 3:18–43. https://doi.org/10.3390/bios3010018
Zhang J, Tian Q, Zhu SF, Zhao WJ, Liu FQ (2012) Rapid on-site detection of Acidovorax citrulli by cross-priming amplification. Mol Cell Probes 26:175–176
Zhang H, Xu Y, Fohlerova Z, Chang H, Iliescu C, Neuzil P (2019a) LAMP-on-a-chip: Revising microfluidic platforms for loop-mediated DNA amplification. Trends Anal Chem 113:44–53
Zhang J, Cao J, Zhu M, Xu M, Feng S (2019b) Loop mediated isothermal amplification-lateral-flow-dipstick (LAMP-LFD) to detect Mycoplasma ovipneumoniae. World J Microbiol Biotechnol 35:31
Zhang J, Di B, Shan H, Liu J, Zhou Y, Chen H, Hu L, Wu X, Bai Z (2019c) Rapid detection of Bacillus cereus using cross-priming amplification. J Food Prot 82(10):1744–1750
Zhang YP, Cui YX, Li XY, Du YC, Tang AN, Kong DM (2019d) A modified exponential amplification reaction (EXPAR) with an improved signal-to-noise ratio for ultrasensitive detection of polynucleotide kinase. Chem Commun 55(53):7611–7614
Zhao Y, Chen F, Li Q, Wang L, Fan C (2015) Isothermal amplification of nucleic acids. Chem Rev 115:12491–12545
Zhao Y, Jiang X, Qu Y, Pan R, Pang X, Jiang Y, Man C (2017) Salmonella detection in powdered dairy products using a novel molecular tool. J Dairy Sci 100(5):3480–3496
Zheng F, Li S, Wang S, Feng T, Jiang Z, Pan J (2020) Cross-priming isothermal amplification combined with nucleic acid test strips for detection of meat species. Anal Biochem 597:113672
Zhong Z, Zhao X (2017) Isothermal amplification technologies for the detection of foodborne pathogens. Food Anal Methods 11:1543–1560
Zhu X, Wang X, Han L, Chen T, Wang L, Li H, Li S, He L, Fu X, Chen S, Xing M, Chen H, Wang Y (2020) Multiplex reverse transcriptase loop mediated isothermal amplification combined with nanoparticle based lateral flow biosensor for the diagnosis of COVID-19. Biosens Bioelectron 166:1–7
Funding
The corresponding author has received grant from the parent institute (Grant Number: GO/Estb/SMS/2019-20/2122).
Author information
Authors and Affiliations
Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. The authors declare the following financial interests/personal relationships which may be considered as potential competing interests.
Human and animal rights
No human/animal has been involved in research and no ethical consent was required.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Srivastava, P., Prasad, D. Isothermal nucleic acid amplification and its uses in modern diagnostic technologies. 3 Biotech 13, 200 (2023). https://doi.org/10.1007/s13205-023-03628-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-023-03628-6