Abstract
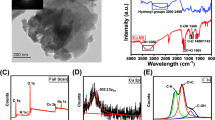
The authors describe a method for the determination of cobalt(II) ions based on the use of luminescent and water-soluble ZnO quantum dots capped with β-cyclodextrin (β-CD@ZnO QDs). The modified QDs display strong yellow-green fluorescence with a peak at 537 nm under 360 nm excitation. High-resolution transmission electron microscopy, Fourier transform infrared spectroscopy, luminescence, and UV-visible absorption spectroscopy were used to characterize the β-CD@ZnO QDs. The fluorescence of the QDs is quenched by Co(II) ions. This finding was exploited to design a quenchometric assay designed for the detection of Co(II) in water solution. The detection limit is 0.34 μM (based on the 3σ/slope criterion), and the linear range extends from 1.0 to 10 μM. The method was applied to quantify Co(II) in spiked real samples. The quenching mechanism was studied, and this showed that aggregation-induced quenching causes the main effect.

The fluorescence of β-cyclodextrin-capped ZnO quantum dots (β-CD@ZnO QDs) is quenched by cobalt ions, and this finding is exploited in a fluorescence assay for cobalt ions in aqueous solutions.






Similar content being viewed by others
References
Zhang J, Zhang R, Zhao LH, Sun SQ (2012) Synthesis of water-soluble γ-aminopropyl triethoxysilane-capped ZnO:MgO nanocrystals with biocompatibility. CrystEngComm 14:613–619
Shim M, Guyot-Sionnest P (2001) Organic-capped ZnO nanocrystals: synthesis and n-type character. J Am Chem Soc 123(47):11651–11654
Xia T, Kovochich M, Liong M, Madler L, Gilbert B, Shi H, Yeh JI, Zink JI, Nel A.E (2008) Comparison of the mechanism of toxicity of zinc oxide and cerium oxide nanoparticles based on dissolution and oxidative stress properties. ACS Nano 2 (10): 2121–2134
Lin KF, Cheng HM, Hsu HC, Lin LJ, Hsieh WF (2005) Band gap variation of size-controlled ZnO quantum dots synthesized by sol–gel method. Chem Phys Lett 409:208–211
Schejn A, Balan L, Piatkowski D, Mackowski S, Lulek J, Schneider R (2012) From visible to white-light emission by siloxane-capped ZnO quantum dots upon interaction with thiols. Opt Mater 34:1357–1361
Xu XY, Xu CX, Wang XM, Lin Y, Dai J, Hu JG (2013) Control mechanism behind broad fluorescence from violet to orange in ZnO quantum dots. CrystEngComm 15:977–981
Jacobsson TJ, Viarbitskaya S, Mukhtar E, Edvinsson T (2014) A size dependent discontinuous decay rate for the exciton emission in ZnO quantum dots. Phys Chem Chem Phys 16:13849–13857
Asok A, Gandhi MN, Kulkarni AR (2012) Enhanced visible photoluminescence in ZnO quantum dots by promotion of oxygen vacancy formation. Nano 4:4943–4946
Zhang J, Wu D, Li MF, Feng J (2015) Multifunctional mesoporous silica nanoparticles based on charge-reversal plug-gate nanovalves and acid-decomposable ZnO quantum dots for intracellular drug delivery. ACS Appl Mater Interfaces 7:26666–26673
Xie QS, Ma YT, Wang XP, Zeng DQ, Wang LS, Mai LQ, Peng DL (2016) Electrostatic assembly of sandwich-like Ag-C@ZnO-C@Ag-C hybrid hollow microspheres with excellent high-rate lithium storage properties. ACS Nano 10(1):1283–1291
Hoffmann MWG, Mayrhofer L, Casals O, Caccamo L, Hernandez-Ramirez F, Lilienkamp G, Daum W, Moseler M, Waag A, Shen H, Prades JD (2014) A highly selective and self-powered gas sensor via organic surface functionalization of p-Si/n-ZnO diodes. Adv Mater 26:8017–8022
Dorfman A, Kumar N, Hahm JI (2006) Nanoscale ZnO-enhanced fluorescence detection of protein interactions. Adv Mater 18:2685–2690
Yuan Q, Hein S, Misra RDK (2010) New generation of chitosan-encapsulated ZnO quantum dots loaded with drug: synthesis, characterization and in vitro drug delivery response. Acta Biomater 6:2732–2739
Sharma H, Singh A, Kaur N, Singh N (2013) ZnO-based imine-linked coupled biocompatible chemosensor for nanomolar detection of Co2+. ACS Sustain Chem Eng 1:1600–1608
Sodzel D, Khranovskyy V, Beni V, Turner APF, Viter R, Eriksson MO, Holtz PO, Janot JM, Bechelany M, Balme S, Smyntyna V, Kolesneva E, Dubovskaya L, Volotovski I, Ubelis A, Yakimova R (2015) Continuous sensing of hydrogen peroxide and glucose via quenching of the UV and visible luminescence of ZnO nanoparticles. Microchim Acta 182:1819–1826
Fu YS, Du XW, Kulinich SA, Qiu JS, Qin WJ, Li R, Sun J, Liu J (2007) Stable aqueous dispersion of ZnO quantum dots with strong blue emission via simple solution route. J Am Chem Soc 129(51):16029–16033
Xiong HM, Xu Y, Ren QG, Xia YY (2008) Stable aqueous ZnO@polymer core-shell nanoparticles with tunable photoluminescence and their application in cell imaging. J Am Chem Soc 130(24):7522–7523
Singh AK, Singh P, Bhattacharjee G (2009) Determination of cobalt ions at nano-level based on newly synthesized pendant armed macrocycle by polymeric membrane and coated graphite electrode. Talanta 80:685–693
Khan SB, Asiri AM, Rahman MM, Marwani HM, Alamry KA (2015) Evaluation of cerium doped tin oxide nanoparticles as a sensitive sensor for selective detection and extraction of cobalt. Phys E 70:203–209
Han Q, Huo YY, Yang N, Yang XH, Hao TT (2015) Determination of cobalt in water by thermal lens spectrometry with cloud point extraction. Anal Lett 48:2096–2106
Bas B, Wegiel K, Jedlinska K (2015) The renewable bismuth bulk annular band working electrode: fabrication and application in the adsorptive stripping voltammetric determination of nickel(II) and cobalt(II). Anal Chim Acta 881:44–53
Awual MR, Yaita T, Shiwaku H, Suzuki S (2015) A sensitive ligand embedded nano-conjugate adsorbent for effective cobalt(II) ions capturing from contaminated water. Chem Eng J 276:1–10
Awual MR, Yaita T, Okamoto Y (2014) A novel ligand based dual conjugate adsorbent for cobalt(II) and copper(II) ions capturing from water. Sensor Actuat B-Chem 203:71–80
Soylak M, Divrikli U, Elci L, Dogan M (2002) Preconcentration of Cr(III), Co(II), Cu(II), Fe(III) and Pb(II) as calmagite chelates on cellulose nitrate membrane filter prior to their flame atomic absorption spectrometric determinations. Talanta 56:565–570
Benkhedda K, Infante HG, Ivanova E, Adams F (2000) Electrothermal atomic absorption spectrometric determination of cobalt in biological samples and natural waters using a flow injection system with on-line preconcentration by ion-pair adsorption in a knotted reactor. Fresenius J Anal Chem 368:288–292
Bispo MS, Morte ESB, Korn MGA, Teixeira LSG, Korn M, Costa ACS (2005) Determination of Pb in river water samples by inductively coupled plasma optical emission spectrometry after ultrasound-assisted co-precipitation with manganese dioxide. Spectrochim Acta B 60:653–658
Ensafi AA, Abbasi S (2000) Highly selective and sensitive stripping voltammetric determination of cobalt with ammonium 2-aminocyclohexene-1-dithiocarboxylate and nitrite. Anal Sci 16:377–381
Heitland P, Koster HD (2004) Fast, simple and reliable routine determination of 23 elements in urine by ICP-MS. J Anal Spectrom 19:1552–1558
Kamath A, Netalkar SP, Kokare DG, Naik K (2012) Revankar V.K., Phenoxide bridged tetranuclear Co(II), Ni(II), Cu(II) and Zn(II) complexes: syntheses, characterization and fluorescence studies. J Lumin 132:2763–2768
Xu S, Li J, Li X, Su M, Shi Z, Zeng Y, Ni S (2016) A chemiluminescence resonance energy transfer system composed of cobalt(II), luminol, hydrogen peroxide and CdTe quantum dots for highly sensitive determination of hydroquinone. Microchim Acta 183:667–673
Sakamoto-Arnold CM, Johnson KS (1987) Determination of picomolar levels of cobalt in seawater by flow injection analysis with chemiluminescence detection. Anal Chem 59(14):1789–1794
Zi LL, Huang Y, Yan ZY, Liao SH (2014) Thioglycolic acid-capped CuInS2/ZnS quantum dots as fluorescent probe for cobalt ion detection. J Lumin 148:359–363
Abdolmaleki A, Mallakpour S, Borandeh S (2014) Tailored functionalization of ZnO nanoparticle via reactive cyclodextrin and its bionanocomposite synthesis. Carbohyd Polym 103:32–37
Peng HX, Cui B, Li GM, Wang YS, Li NN, Chang ZG, Wang YY (2015) A multifunctional beta-CD-modified Fe3O4@ZnO:Er3+, Yb3+ nanocarrier for antitumor drug delivery and microwave-triggered drug release. Mat Sci Eng C-Mater 46:253–263
Geng S, Lin SM, Liu SG, Li NB, Luo HQ (2016) A new fluorescent sensor for detecting p-nitrophenol based on β-cyclodextrin-capped ZnO quantum dots. RSC Adv 6:86061–86067
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 21675131) and the Municipal Science Foundation of Chongqing City (No. CSTC-2015jcyjB50001).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOC 344 kb)
Rights and permissions
About this article
Cite this article
Geng, S., Lin, S.M., Shi, Y. et al. Determination of cobalt(II) using β-cyclodextrin-capped ZnO quantum dots as a fluorescent probe. Microchim Acta 184, 2533–2539 (2017). https://doi.org/10.1007/s00604-017-2193-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-017-2193-4