Abstract
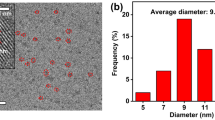
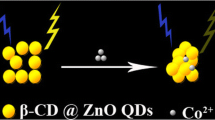
A new fluorescence sensor for Ce3+ions is reported in this paper. This sensor is based on the fluorescence quenching of glycine dithiocarbamate (GDTC)-functionalized manganese doped ZnS quantum dots (QDs) in the presence of Ce3+ions. The synthesis of ultra-small GDTC-Mn:ZnS quantum dots (QDs) is based on the co-precipitation of nanoparticles in aqueous Solution. The nanoparticles are characterized with fluorescence spectroscopy, UV–vis absorption spectra, high-resolution transmission electron microscopy, X-ray power diffraction (XRD), and infrared spectroscopy. In the test carried out, it was found that the interaction between Ce3+ions and GDTC capped Mn:ZnS QDs quenches the original fluorescence of QDs according to the Stern-Volmer equation and the results show the existence of collisional quenching process. A linear relationship was observed between the extent of quenching and the concentration of Ce3+in the range of 2.0 × 10−6 to 3.2 × 10−5 mol.L−1, with a detection limit of 2.29 × 10−7 mol.L−1. The relative standard deviation of 1.61 % was obtained for five replicate measurements. The possible quenching mechanism was also examined by fluorescence and UV–vis absorption spectra. The interference of other cations was negligible on the quantitative determination of Ce3+. This method proved to be simple, sensitive, low cost, and also reliable for practical applications.















Similar content being viewed by others
References
Kuang H, Zhao Y, Ma W, Xu L, Wang L, Xu C (2011) Recent developments in analytical applications of quantum dots. Trends Anal Chem 30:1620–1636
Gill R, Zayats M, Willner I (2008) Semiconductor quantum dots for bioanalysis. Angew Chem Int Ed 40:7602–7625
Rossetti R, Nakahara S, Brus LE (1983) Quantum size effects in the redox potentials, resonance Raman spectra, and electronic spectra of CdS crystallites in aqueous solution. J Chem Phys 79:1086–1088
Callan JF, De Silva AP, Mulrooney RC, Mc Caughan B (2007) Luminescent sensing with quantum dots. J Fluoresc 3–4:257–262
Galian RE, Guardia M (2009) The use of quantum dots in organic chemistry. Trends Anal Chem 28:279–91
Freeman R, Willner I (2012) Optical molecular sensing with semiconductor quantum dots (QDs). Chem Soc Rev 41:4067–85
Thomas D, Lonappan L, Rajith L, Cyriac ST, Kumar KG (2013) Quantum Dots (QDs) Based Fluorescent Sensor for the Selective Determination of Nimesulide. J Fluoresc 3:473–478
Jian W, Zhuang J, Zhang D, Dai J, Yang W, Bai Y (2006) Synthesis of highly luminescent and photostable ZnS:Ag nanocrystals under microwave irradiation. Mater Chem Phys 99:494–7
Labiadh H, Chaabane TB, Piatkowski D, Mackowski S, Lalevée J, Ghanbaja J et al (2013) Aqueous route to color-tunable Mn-doped ZnS quantum dots. Mater Chem Phys 140:674–682
Khosravi AA, Kundu M, Jatwa L, Deshpande SK, Bhagwat UA, Sastry M et al (1995) Green luminescence from copper doped zinc sulphide quantum particles. Appl Phys Lett 67:2702–2704
Qu SC, Zhou WH, Liu FQ, Chen NF, Wang ZG, Pan HY et al (2002) Photoluminescence properties of Eu3+-doped ZnS nanocrystals prepared in a water/methanol solution. Appl Phys Lett 80:3605–7
Suyver JF, Wuister SF, Kelly JJ, Meijerink A (2001) Synthesis and photoluminescence of nanocrystalline ZnS:Mn2+. Nano Lett 1:429–33
Ma X, Song J, Yu Z (2011) The light emission properties of ZnS:Mn nanoparticles. Thin Solid Films 519:5043–5045
Segets D, Komada S, Butz B, Spiecker E, Mori Y, Peukert W (2013) Quantitative evaluation of size selective precipitation of Mn-doped ZnS quantum dots by size distributions calculated from UV/Vis absorbance spectra. J Nanoparticle Res 15:1–13
Ali TA, Mohamed GG, Azzam EMS, Abd-elaal AA (2014) Thiol surfactant assembled on gold nanoparticles ion exchanger for screen-printed electrode fabrication Potentiometric determination of Ce(III) in environmental polluted samples. Sensors Actuators B Chem 191:192–203
Afkhami A, Madrakian T, Shirzadmehr A, Tabatabaee M, Bagheri H (2012) New Schiff base-carbon nanotube–nanosilica–ionic liquid as a high performance sensing material of a potentiometric sensor for nanomolar determination of cerium(III) ions. Sensors Actuators B Chem 174:237–44
Awual MR, Yaita T, Shiwaku H (2013) Design a novel optical adsorbent for simultaneous ultra-trace cerium (III) detection, sorption and recovery. Chem Eng J 228:327–35
Wang L, Yu Y, Huang X, Long Z, Cui D (2013) Toward greener comprehensive utilization of bastnaesite: Simultaneous recovery of cerium, fluorine, and thorium from bastnaesite leach liquor using HEH(EHP). Chem Eng J 215–216:162–167
Abhilash S, Sinha MK, Sinha BDP (2014) Extraction of lanthanum and cerium from Indian red mud. Int J Miner Process 127:70–73
Hirano S, Suzuki KT (1996) Exposure, metabolism, and toxicity of rare earths and related compounds. Environ Health Perspect 104:85–95
Masi AN, Olsina RA (1993) Preconcentration and determination of Ce, La and Pr by X-ray fluorescence analysis, using Amberlite XAD resins loaded with 8-Quinolinol and 2-(2-(5 chloropyridylazo)-5-dimethylamino)-phenol. Talanta 40:931–934
Achilli M, Ciceri G, Ferraroli R, Heltai D, Martinotti W (1989) Determination of cerium, yttrium and thorium in environmental samples. Analyst 114:319–23
Ayranov M, Cobos J, Popa K, Rondinella VV (2009) Determination of REE, U, Th, Ba, and Zr in simulated hydrogeological leachates by ICP-AES after matrix solvent extraction. J Rare Earths 27:123–7
Amin AS, Moustafa MM, Issa RM (1997) A rapid, selective and sensitive spectrophotometric method for the determination of Ce(III) using some bisazophenyl-β-diketone derivatives. Talanta 44:311–317
Zhang T, Lu J, Ma J, Qiang Z (2008) Fluorescence spectroscopic characterization of DOM fractions isolated from a filtered river water after ozonation and catalytic ozonation. Chemosphere 71:911–21
Meng JX, Wu HJ, Feng DX (2000) Fluorimetry determination of trace Ce3+ with EDTP. Spectrochim Acta A 56:1925–8
Rounaghi G, Zadeh Kakhki RM, Sadeghian H (2011) A new cerium (III) ion selective electrode based on 2,9-dihydroxy-1,10-diphenoxy-4,7-dithia decane, a novel synthetic ligand. Electrochim Acta 56:9756–9761
Gupta VK, Singh AK, Gupta B (2006) A cerium(III) selective polyvinyl chloride membrane sensor based on a Schiff base complex of N, N′-bis[2-(salicylideneamino)ethyl]ethane-1,2-diamine. Anal Chim Acta 575:198–204
Akseli A, Rakicioğlu Y (1996) Fluorimetric trace determination of cerium(III) with sodium triphosphate. Talanta 43:1983–1988
Zhang Y, Schnoes AM, Clapp AR (2010) Dithiocarbamates as Capping Ligands for Water-Soluble Quantum Dots. ACS Appl Mater Interfaces 2:3384–3395
Thirumaran S, Ramalingam K (2000) Mixed ligand complexes involving aminoacid dithiocarbamates, substituted phosphines and nickel(II). Transit Met Chem 25:60–62
Criado JJ, Carrasco A, Macias B, Salas JM, Medarde M, Castillo M (1989) New PtS4 chromophores of dithiocarbamates derived from α-amino acids: synthesis, characterization and thermal behaviour. Inorg Chim Acta 160:37–42
Rajabi HR, Shamsipur M, Khosravi AA, Khani O, Yousefi MH (2013) Selective spectrofluorimetric determination of sulfide ion using manganese doped ZnS quantum dots as luminescent probe. Spectrochim Acta A 107:256–62
Wu H, Fan Z (2012) Mn-doped ZnS quantum dots for the room-temperature phosphorescence detection of raceanisodamine hydrochloride and atropine sulfate in biological fluids. Spectrochim Acta A 90:131–134
Bhargava RN (1996) Doped nanocrystalline materials-Physics and applications. J Lumin 70:85–94
Cao J, Yang J, Zhang Y, Yang L, Wang Y, Wei M et al (2009) Optimized doping concentration of manganese in zinc sulfide nanoparticles for yellow-orange light emission. J Alloys Compd 486:890–894
Ren Z, Yang H, Shen L, Han S (2008) Hydrothermal preparation and properties of nanocrystalline ZnS:Mn. J Mater Sci Mater Electron 19:1–4
Viswanath R, Bhojya Naik HS, Yashavanth Kumar GS, Prashanth Kumar PN, Harish KN, Prabhakara MC et al (2014) Synthesis and photoluminescence enhancement of PVA capped Mn2+ doped ZnS nanoparticles and observation of tunable dual emission: a new approach. Appl Surf Sci 301:126–33
Tauc J, Menth A (1972) States in the gap. J Non-Cryst Solids 8–10:569–85
Chopra N, Mansingh A, Chadha GK (1990) Electrical, optical and structural properties of amorphous V2O5-TeO2 blown films. J Non-Cryst Solids 126:194–201
Ghobadi N (2013) Band gap determination using absorption spectrum fitting procedure. Int Nano Lett 3:2
Mu J, Gu D, Xu Z (2005) Synthesis and stabilization of ZnS nanoparticles embedded in silica nanospheres. Appl Phys A 80:1425–1429
Kole AK, Tiwary CS, Kumbhakar P (2013) Room temperature synthesis of Mn2+ doped ZnS d-dots and observation of tunable dual emission: Effects of doping concentration, temperature, and ultraviolet light illumination. J Appl Phys 113:114308
Dong B, Cao L, Su G, Liu W (2012) Synthesis and characterization of Mn doped ZnS d-dots with controllable dual-color emissions. J Colloid Interface Sci 367:178–182
Criado JJ, Fernandez I, Macias B, Salas JM, Medarde M (1990) Novel chelates of Pd(II) dithiocarbamates. Spectroscopic studies and thermal behaviour. Inorg Chim Acta 174:67–75
Stewart MH, Susumu K, Mei BC, Medintz IL, Delehanty JB, Blanco-Canosa JB et al (2010) Multidentate Poly(ethylene glycol) ligands provide colloidal stability to semiconductor and metallic nanocrystals in extreme conditions. J Am Chem Soc 132:9804–9813
Geszke-Moritz M, Clavier G, Lulek J, Schneider R (2012) Copper- or manganese-doped ZnS quantum dots as fluorescent probes for detecting folic acid in aqueous media. J Lumin 132:987–91
Aldana J, Lavelle N, Wang Y, Peng X (2005) Size-dependent dissociation ph of thiolate ligands from cadmium chalcogenide nanocrystals. J Am Chem Soc 127:2496–2504
Gao M, Kirstein S, Möhwald H, Rogach AL, Kornowski A, Eychmüller A et al (1998) Strongly photoluminescent CdTe nanocrystals by proper surface modification. J Phys Chem B 102:8360–8363
Koneswaran M, Narayanaswamy R (2009) l-Cysteine-capped ZnS quantum dots based fluorescence sensor for Cu2+ ion. Sensors Actuators B Chem 139:104–109
Wang J, Xu J, Goodman MD, Chen Y, Cai M, Shinar J et al (2008) A simple biphasic route to water soluble dithiocarbamate functionalized quantum dots. J Mater Chem 18:3270–4
Querner C, Reiss P, Bleuse J, Pron A (2004) Chelating ligands for nanocrystals’ surface functionalization. J Am Chem Soc 126:11574–11582
Aldana J, Wang YA, Peng X (2001) Photochemical instability of CdSe nanocrystals coated by hydrophilic thiols. J Am Chem Soc 12:8844–8850
Zamani HA, Ganjali MR, Adib M (2007) Construction of a highly selective PVC-based membrane sensor for Ce(III) ions. Sensors Actuators B Chem 120:545–550
Jain VK, Handa A, Sait SS, Shrivastav P, Agrawal YK (2001) Pre-concentration, separation and trace determination of lanthanum(III), cerium(III), thorium(IV) and uranium(VI) on polymer supported o-vanillin semicarbazone. Anal Chim Acta 429:237–246
Wu P, Zhao T, Wang S, Hou X (2014) Semicondutor quantum dots-based metal ion probes. Nanoscale 6:43–64
Duan J, Jiang X, Ni S, Yang M, Zhan J (2011) Facile synthesis of N-acetyl-l-cysteine capped ZnS quantum dots as an eco-friendly fluorescence sensor for Hg2+. Talanta 85:1738–1743
Zhang K, Guo J, Nie J, Du B, Xu D (2014) Ultrasensitive and selective detection of Cu2+ in aqueous solution with fluorescence enhanced CdSe quantum dots. Sensors Actuators B Chem 190:279–287
Chen J, Gao Y, Guo C, Wu G, Chen Y, Lin B (2008) Facile synthesis of water-soluble and size-homogeneous cadmium selenide nanoparticles and their application as a long-wavelength fluorescent probe for detection of Hg(II) in aqueous solution. Spectrochim Acta Mol Biomol Spectros 69:572–579
Kobayashi S, Sugiura M, Kitagawa H, Lam WWL (2002) Rare-earth metal triflates in organic synthesis. Chem Rev 102:2227–2302
Pearson RG (1963) Hard and soft acids and bases. J Am Chem Soc 85:3533–3539
Cai ZX, Yang H, Zhang Y, Yan XP (2006) Preparation, characterization and evaluation of water-soluble l-cysteine-capped-CdS nanoparticles as fluorescence probe for detection of Hg(II) in aqueous solution. Anal Chim Acta 559:234–239
Shanmugam N, Cholan S, Viruthagiri G, Gobi R, Kannadasan N (2014) Synthesis and characterization of Ce3+-doped flowerlike ZnS nanorods. Appl Nanosci 4:359–365
Wang J, Zhou X, Ma H, Tao G (2011) Diethyldithiocarbamate functionalized CdSe/CdS quantum dots as a fluorescent probe for copper ion detection. Spectrochim Acta Mol Biomol Spectros 81:178–183
Acknowledgments
The authors are thankful to the Chemistry and Chemical Engineering Research Center of Iran for providing departmental facilities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rofouei, M.K., Tajarrod, N., Masteri-Farahani, M. et al. A New Fluorescence Sensor for Cerium (III) Ion Using Glycine Dithiocarbamate Capped Manganese Doped ZnS Quantum Dots. J Fluoresc 25, 1855–1866 (2015). https://doi.org/10.1007/s10895-015-1678-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-015-1678-y