Abstract
The authors describe the synthesis of a multifunctional nanocomposite with an architecture of type Fe3O4@SiO2@graphene quantum dots with an average diameter of about 22 nm. The graphene quantum dots (GQDs) were covalently immobilized on the surface of silica-coated magnetite nanospheres via covalent linkage to surface amino groups. The nanocomposite displays a strong fluorescence (with excitation/emission peaks at 330/420 nm) that is fairly selectively quenched by Hg2+ ions, presumably due to nonradiative electron/hole recombination annihilation. Under the optimized experimental conditions, the linear response to Hg2+ covers the 0.1 to 70 μM concentration range, with a 30 nM lower detection limit. The high specific surface area and abundant binding sites of the GQDs result in a good adsorption capacity for Hg2+ (68 mg⋅g−1). The material, due to its superparamagnetism, can be separated by using a magnet and also is recyclable with EDTA so that it can be repeatedly used for simultaneous detection and removal of Hg2+ from contaminated water.

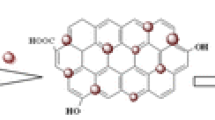
A schematic view of preparation process for the Fe3O4@SiO2@graphene quantum dots nanocomposite (denoted as Fe3O4@SiO2@GQDs). The graphene quantum dots were covalently immobilized on the surface of silica-coated magnetite nanospheres (Fe3O4@SiO2) via covalent linkage to surface amino groups.





Similar content being viewed by others
References
Amini MK, Khezri B, Firooz AR (2008) Development of a highly sensitive and selective optical chemical sensor for batch and flow-through determination of mercury ion. Sensors Actuators B Chem 131:470–478
Wang ZX, Ding SN (2014) One-pot green synthesis of high quantum yield oxygen-doped, nitrogen-rich, photoluminescent polymer carbon nanoribbons as an effective fluorescent sensing platform for sensitive and selective detection of silver(I) and mercury(II) ions. Anal Chem 86:7436–7445
Ding Y, Wang S, Li J, Chen L (2016) Nanomaterial-based optical sensors for mercury ions. Trends Anal Chem 82:175–190
Hallaj T, Amjadi M, Manzoori JL, Shokri R (2015) Chemiluminescence reaction of glucose-derived graphene quantum dots with hypochlorite, and its application to the determination of free chlorine. Microchim Acta 182:789–796
Li Y, Hu Y, Zhao Y, Shi G, Deng L, Hou Y, Qu L (2011) An electrochemical avenue to green luminescent graphene quantum dots as potential electron-acceptors for photovoltaics. Adv Mater 23:776–780
Sun R, Wang Y, Ni Y, Kokot S (2014) Graphene quantum dots and the resonance light scattering technique for trace analysis of phenol in different water samples. Talanta 125:341–346
Bacon M, Bradley SJ, Nann T (2014) Graphene quantum dots. Part Part Syst Charact 31:415–428
Li L, Wu G, Yang G, Peng J, Zhao J, Zhu JJ (2013) Focusing on luminescent graphene quantum dots: current status and future perspectives. Nanoscale 5:4015–4039
Xu J, Wang Y, Hu S (2017) Nanocomposites of graphene and graphene oxides: synthesis, molecular functionalization and application in electrochemical sensors and biosensors. A review. Microchim Acta 184:1–44
Zhang C, Cui Y, Song L, Liu X, Hu Z (2016) Microwave assisted one-pot synthesis of graphene quantum dots as highly sensitive fluorescent probes for detection of iron ions and pH value. Talanta 150:54–60
Wang FX, Gu ZY, Lei W, Wang WJ, Xia XF, Hao QL (2014) Graphene quantum dots as a fluorescent sensing platform for highly efficient detection of copper(II) ions. Sensors Actuators B Chem 190:516–522
Qi YX, Zhang M, Fu QQ, Liu R, Shi GY (2013) Highly sensitive and selective fluorescent detection of cerebral lead(II) based on graphene quantum dot conjugates. Chem Commun 49:10599–10601
Chakraborti H, Sinha S, Ghosh S, Pal SK (2013) Interfacing water soluble nanomaterials with fluorescence chemosensing: graphene quantum dot to detect Hg2+ in 100% aqueous solution. Mater Lett 97:78–80
Wang B, Zhuo S, Chen L, Zhang Y (2014) Fluorescent graphene quantum dot nanoprobes for the sensitive and selective detection of mercury ions. Spectrochim Acta A Mol Biomol Spectrosc 131:384–387
Li Z, Wang Y, Ni Y, Kokot S (2015) A rapid and label-free dual detection of Hg (II) and cysteine with the use of fluorescence switching of graphene quantum dots. Sensors Actuators B Chem 207:490–497
Yu N, Peng H, Xiong H, Wu X, Wang X, Li Y, Chen L (2015) Graphene quantum dots combined with copper(II) ions as a fluorescent probe for turn-on detection of sulfide ions. Microchim Acta 182:2139–2146
Zhou Y, Qu ZB, Zeng Y, Zhou T, Shi G (2014) A novel composite of graphene quantum dots and molecularly imprinted polymer for fluorescent detection of paranitrophenol. Biosens Bioelectron 52:317–323
Shao T, Zhang P, Tang L, Zhuo S, Zhu C (2015) Highly sensitive enzymatic determination of urea based on the pH-dependence of the fluorescence of graphene quantum dots. Microchim Acta 182:1431–1437
He YZ, Wang XX, Sun J, Jiao SF, Chen HQ, Gao F, Wang L (2014) Fluorescent blood glucose monitor by hemin-functionalized grapheme quantum dots based sensing system. Anal Chim Acta 810:71–78
Xu Y, Zhou Y, Ma W, Wang S, Li S (2013) Functionalized magnetic core–shell Fe3O4@SiO2 nanoparticles for sensitive detection and removal of Hg2+. J Nanopart Res 15:1716–1724
Xiao D, Lu T, Zeng R, Bi Y (2016) Preparation and highlighted applications of magnetic microparticles and nanoparticles: a review on recent advances. Microchim Acta 183:2655–2675
Lu D, Yang L, Tian Z, Wang L, Zhang J (2012) Core-shell mesoporous silica nanospheres used as Zn2+ ratiometric fluorescent sensor and adsorbent. RSC Adv 2:2783–2789
Dong Y, Shao J, Chen C, Li H, Wang R, Chi Y, Lin X, Chen G (2012) Blue luminescent graphene quantum dots and graphene oxide prepared by tuning the carbonization degree of citric acid. Carbon 50:4738–4743
Liu Y, Liu CY, Zhang ZY (2011) Synthesis and surface photochemistry of graphitized carbon quantum dots. J Colloid Interface Sci 356:416–421
Dai Y, Long H, Wang X, Wang Y, Gu Q, Jiang W, Wang Y, Li C, Zeng TH, Sun Y, Zeng J (2014) Versatile graphene quantum dots with tunable nitrogen doping. Part Part Syst Charact 31:597–604
Fan LS, Hu YW, Wang X, Zhang LL, Li FH, Han DX, Li ZG, Zhang QX, Wang ZX, Niu L (2012) Fluorescence resonance energy transfer quenching at the surface of graphene quantum dots for ultrasensitive detection of TNT. Talanta 101:192–197
Lakowicz JR (2006) Principles of fluorescence spectroscopy. Springer, New York
Mohapatra S, Sahu S, Sinha N, Bhutia SK (2015) Synthesis of a carbon-dot-based photoluminescent probe for selective and ultrasensitive detection of Hg2+ in water and living cells. Analyst 140:1221–1228
Lu Y, Yu J, Ye W, Yao X, Zhou P, Zhang H, Zhao S, Jia L (2016) Spectrophotometric determination of mercury(II) ions based on their stimulation effect on the peroxidase-like activity of molybdenum disulfide nanosheets. Microchim Acta 183:2481–2489
Kamali KZ, Pandikumar A, Jayabal S, Ramaraj R, Lim HN, Ong BH, Bien CSD, Kee YY, Huang NM (2016) Amalgamation based optical and colorimetric sensing of mercury(II) ions with silver@graphene oxide nanocomposite materials. Microchim Acta 183:369–377
Chen ZB, Zhang CM, Tan Y, Zhou TH, Ma H, Wan CQ, Lin YQ, Li K (2015) Chitosan-functionalized gold nanoparticles for colorimetric detection of mercury ions based on chelation-induced aggregation. Microchim Acta 182:611–616
Shamsipur M, Safavi A, Mohammadpour Z, Ahmadi R (2016) Highly selective aggregation assay for visual detection of mercury ion based on competitive binding of sulfur-doped carbon nanodots to gold nanoparticles and mercury ions. Microchim Acta 183:2327–2335
Yan F, Kong D, Luo Y, Ye Q, He J, Guo X, Chen L (2016) Carbon dots serve as an effective probe for the quantitative determination and for intracellular imaging of mercury(II). Microchim Acta 183:1611–1618
Yang R, Ding X, Zhou Y, Li J, Qu L, Zhang K (2015) A novel fluorescent sensor for mercury (II) ion using self-assembly of poly(diallyl dimethyl ammonium)chloride functionalized CdTe quantum dots. Anal Methods 7:436–442
Ding X, Qu L, Yang R, Zhou Y, Yang J, Li J (2015) A highly selective and simple fluorescent sensor for mercury (II) ion detection based on cysteamine-capped CdTe quantum dots synthesized by the reflux method. Luminescence 30:465–471
Zhang J, Yu SH (2014) Highly photoluminescent silicon nanocrystals for rapid, label-free and recyclable detection of mercuric ions. Nanoscale 6:4096–4101
Lu W, Qin X, Liu S, Chang G, Zhang Y, Luo Y, Asiri AM, Alyoubi AO, Sun X (2012) Economical, green synthesis of fluorescent carbon nanoparticles and their use as probes for sensitive and selective detection of mercury(II) ions. Anal Chem 84:5351–5357
Wu ZZ, Li WY, Chen J, Yu C (2014) A graphene quantum dot-based method for the highly sensitive and selective fluorescence turn on detection of biothiols. Talanta 119:538–543
Xia YS, Zhu CQ (2008) Use of surface-modified CdTe quantum dots as fluorescent probes in sensing mercury (II). Talanta 75:215–221
Foo KY, Hameed BH (2010) Insights into the modeling of adsorption isotherm systems. Chem Eng J 156:2–10
Acknowledgements
We gratefully acknowledge financial support of this investigation by The Research Council of University of Tehran through Grant (Grant no.94009890).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOCX 946 kb)
Rights and permissions
About this article
Cite this article
Alvand, M., Shemirani, F. A Fe3O4@SiO2@graphene quantum dot core-shell structured nanomaterial as a fluorescent probe and for magnetic removal of mercury(II) ion. Microchim Acta 184, 1621–1629 (2017). https://doi.org/10.1007/s00604-017-2134-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-017-2134-2