Abstract
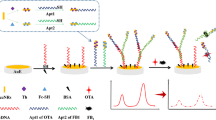
The authors describe an amperometric aptasensor for the mycotoxin ochrotoxin A (OTA). It is based on the use of a modified gold electrode containing aptamer (Apt) as the sensing ligand, Methylene Blue (MB) as the redox indicator, single-walled carbon nanotubes (SWCNTs) as electrochemical signal amplifiers, and complementary strands of aptamer (CSs) as assisting DNA. In the absence of OTA, the duplex formed between Apt and CSs on the electrode remains intact. Thus, a strong electrochemical signal is observed due to the presence of the redox marker MB in the duplex. If OTA is added, the duplex will be disassembled and MB and SWCNTs will be released from the surface of the gold electrode. Hence, the electrochemical signal is weakened. The method is highly specificity for OTA and has a limit of detection as low as 52 pM. The aptasensor was successfully applied to the determination of OTA in (spiked) serum and grape juice samples where it shows LODs of 134 and 58 pM, respectively.





Similar content being viewed by others
References
Wang C, Qian J, Wang K, Liu Q, Dong X, Huang X (2015) Magnetic-fluorescent-targeting multifunctional aptasensorfor highly sensitive and one-step rapid detection of ochratoxin a. Biosens Bioelectron 68:783–790. doi:10.1016/j.bios.2015.02.008
Wei Y, Zhang J, Wang X, Duan Y (2015) Amplified fluorescent aptasensor through catalytic recycling for highly sensitive detection of ochratoxin a. Biosens Bioelectron 65:16–22. doi:10.1016/j.bios.2014.09.100
Sanzani SM, Reverberi M, Fanelli C, Ippolito A (2015) Detection of ochratoxin a using molecular beacons and real-time PCR thermal cycler. Toxins 7(3):812–820. doi:10.3390/toxins7030812
Yang M, Jiang B, Xie J, Xiang Y, Yuan R, Chai Y (2014) Electrochemiluminescence recovery-based aptasensor for sensitive ochratoxin a detection via exonuclease-catalyzed target recycling amplification. Talanta 125:45–50. doi:10.1016/j.talanta.2014.02.061
Zhu Z, Feng M, Zuo L, Wang F, Chen L, Li J, Shan G, Luo SZ (2015) An aptamer based surface plasmon resonance biosensor for the detection of ochratoxin a in wine and peanut oil. Biosens Bioelectron 65:320–326. doi:10.1016/j.bios.2014.10.059
Chen J, Zhang X, Cai S, Wu D, Chen M, Wang S, Zhang J (2014) A fluorescent aptasensor based on DNA-scaffolded silver-nanocluster for ochratoxin a detection. Biosens Bioelectron 57:226–231. doi:10.1016/j.bios.2014.02.001
Lv L, Cui C, Liang C, Quan W, Wang S, Guo Z (2016) Aptamer-based single-walled carbon nanohorn sensors for ochratoxin a detection. Food Control 60:296–301. doi:10.1016/j.foodcont.2015.08.002
Wang C, Dong X, Liu Q, Wang K (2015) Label-free colorimetric aptasensor for sensitive detection of ochratoxin a utilizing hybridization chain reaction. Anal Chim Acta 860:83–88. doi:10.1016/j.aca.2014.12.031
Lin X, Leung KH, Lin L, Lin S, Leung CH, Ma DL, Lin JM (2016) Determination of cell metabolite VEGF165 and dynamic analysis of protein-DNA interactions by combination of microfluidic technique and luminescent switch-on probe. Biosens Bioelectron 79:41–47. doi:10.1016/j.bios.2015.11.089
Huo Y, Qi L, Lv XJ, Lai T, Zhang J, Zhang ZQ (2016) A sensitive aptasensor for colorimetric detection of adenosine triphosphate based on the protective effect of ATP-aptamer complexes on unmodified gold nanoparticles. Biosens Bioelectron 78:315–320. doi:10.1016/j.bios.2015.11.043
Reinemann C, Freiin von Fritsch U, Rudolph S, Strehlitz B (2016) Generation and characterization of quinolone-specific DNA aptamers suitable for water monitoring. Biosens Bioelectron 77:1039–1047. doi:10.1016/j.bios.2015.10.069
Yang F, Wang P, Wang R, Zhou Y, Su X, He Y, Shi L, Yao D (2016) Label free electrochemical aptasensor for ultrasensitive detection of ractopamine. Biosens Bioelectron 77:347–352. doi:10.1016/j.bios.2015.09.050
Ramezani M, Danesh NM, Lavaee P, Abnous K, Taghdisi SM (2016) A selective and sensitive fluorescent aptasensor for detection of kanamycin based on catalytic recycling activity of exonuclease III and gold nanoparticles. Sensors Actuators B Chem 222:1–7. doi:10.1016/j.snb.2015.08.024
Li H, Qiao Y, Li J, Fang H, Fan D, Wang W (2016) A sensitive and label-free photoelectrochemical aptasensor using Co-doped ZnO diluted magnetic semiconductor nanoparticles. Biosens Bioelectron 77:378–384. doi:10.1016/j.bios.2015.09.066
Wang B, Chen Y, Wu Y, Weng B, Liu Y, Lu Z, Li CM, Yu C (2016) Aptamer induced assembly of fluorescent nitrogen-doped carbon dots on gold nanoparticles for sensitive detection of AFB1. Biosens Bioelectron 78:23–30. doi:10.1016/j.bios.2015.11.015
Jo EJ, Mun H, Kim SJ, Shim WB, Kim MG (2016) Detection of ochratoxin a (OTA) in coffee using chemiluminescence resonance energy transfer (CRET) aptasensor. Food Chem 194:1102–1107. doi:10.1016/j.foodchem.2015.07.152
Emrani AS, Danesh NM, Ramezani M, Taghdisi SM, Abnous K (2016) A novel fluorescent aptasensor based on hairpin structure of complementary strand of aptamer and nanoparticles as a signal amplification approach for ultrasensitive detection of cocaine. Biosens Bioelectron 79:288–293. doi:10.1016/j.bios.2015.12.025
Zheng Y, Yuan Y, Chai Y, Yuan R (2015) A label-free electrochemical aptasensor based on the catalysis of manganese porphyrins for detection of thrombin. Biosens Bioelectron 66:585–589. doi:10.1016/j.bios.2014.12.022
Miao Y, Gan N, Li T, Zhang H, Cao Y, Jiang Q (2015) A colorimetric aptasensor for chloramphenicol in fish based on double-stranded DNA antibody labeled enzyme-linked polymer nanotracers for signal amplification. Sensors Actuators B Chem 220:679–687. doi:10.1016/j.snb.2015.05.106
Emrani AS, Danesh NM, Lavaee P, Ramezani M, Abnous K, Taghdisi SM (2016) Colorimetric and fluorescence quenching aptasensors for detection of streptomycin in blood serum and milk based on double-stranded DNA and gold nanoparticles. Food Chem 190:115–121. doi:10.1016/j.foodchem.2015.05.079
Jain A, Homayoun A, Bannister CW, Yum K (2015) Single-walled carbon nanotubes as near-infrared optical biosensors for life sciences and biomedicine. Biotechnol J 10(3):447–459. doi:10.1002/biot.201400168
Ajori S, Ansari R (2015) Vibrational characteristics of diethyltoluenediamines (DETDA) functionalized carbon nanotubes using molecular dynamics simulations. Phys B Condens Matter 459:58–61. doi:10.1016/j.physb.2014.11.101
Miller TS, Sansuk S, Epei S, Lai SCS, Macpherson JV, Unwin PR (2015) Pt nanoparticle modified single walled carbon nanotube network electrodes for electrocatalysis: control of the specific surface area over three orders of magnitude. Catal Today 244:136–145. doi:10.1016/j.cattod.2014.06.022
Taghdisi SM, Danesh NM, Emrani AS, Ramezani M, Abnous K (2015) A novel electrochemical aptasensor based on single-walled carbon nanotubes, gold electrode and complimentary strand of aptamer for ultrasensitive detection of cocaine. Biosens Bioelectron 73:245–250. doi:10.1016/j.bios.2015.05.065
Hu K, Huang Y, Wang S, Zhao S (2014) A carbon nanotubes based fluorescent aptasensor for highly sensitive detection of adenosine deaminase activity and inhibitor screening in natural extracts. J Pharm Biomed Anal 95:164–168. doi:10.1016/j.jpba.2014.02.027
Bai HY, Campo FJ, Tsai YC (2013) Sensitive electrochemical thrombin aptasensor based on gold disk microelectrode arrays. Biosens Bioelectron 42(1):17–22
Zhou L, Wang J, Li D, Li Y (2014) An electrochemical aptasensor based on gold nanoparticles dotted graphene modified glassy carbon electrode for label-free detection of bisphenol a in milk samples. Food Chem 162:34–40
Taghdisi SM, Danesh NM, Ramezani M, Emrani AS, Abnous K (2016) A novel electrochemical aptasensor based on Y-shape structure of dual-aptamer-complementary strand conjugate for ultrasensitive detection of myoglobin. Biosens Bioelectron 80:532–537. doi:10.1016/j.bios.2016.02.029
Mokhtarzadeh A, Ezzati Nazhad Dolatabadi J, Abnous K, de la Guardia M, Ramezani M (2015) Nanomaterial-based cocaine aptasensors. Biosens Bioelectron 68:95–106. doi:10.1016/j.bios.2014.12.052
Taghdisi SM, Danesh NM, Ramezani M, Abnous K (2016) A novel M-shape electrochemical aptasensor for ultrasensitive detection of tetracyclines. Biosens Bioelectron 85:509–514. doi:10.1016/j.bios.2016.05.048
Abnous K, Danesh NM, Ramezani M, Taghdisi SM, Emrani AS (2016) A novel electrochemical aptasensor based on H-shape structure of aptamer-complimentary strands conjugate for ultrasensitive detection of cocaine. Sensors Actuators B Chem 224:351–355. doi:10.1016/j.snb.2015.10.039
Nameghi MA, Danesh NM, Ramezani M, Hassani FV, Abnous K, Taghdisi SM (2016) A fluorescent aptasensor based on a DNA pyramid nanostructure for ultrasensitive detection of ochratoxin a. Anal Bioanal Chem 408(21):5811–5818. doi:10.1007/s00216-016-9693-7
Wang R, Xiang Y, Zhou X, Liu LH, Shi H (2015) A reusable aptamer-based evanescent wave all-fiber biosensor for highly sensitive detection of ochratoxin a. Biosens Bioelectron 66:11–18. doi:10.1016/j.bios.2014.10.079
Mishra RK, Hayat A, Catanante G, Istamboulie G, Marty JL (2016) Sensitive quantitation of ochratoxin a in cocoa beans using differential pulse voltammetry based aptasensor. Food Chem 192:799–804. doi:10.1016/j.foodchem.2015.07.080
Huang X, Li Y, Chen Y, Gao W (2015) Combining a loop-stem aptamer sequence with methylene blue: a simple assay for thrombin detection by resonance light scattering technique. RSC Adv 5(38):30268–30274. doi:10.1039/c4ra14729d
Lee CY, Wu KY, Su HL, Hung HY, Hsieh YZ (2013) Sensitive label-free electrochemical analysis of human IgE using an aptasensor with cDNA amplification. Biosens Bioelectron 39(1):133–138. doi:10.1016/j.bios.2012.07.009
Zhang Z, Wang X, Wang Y, Yang X (2010) Distinction of single base mismatches in duplex DNA using methylene blue as optical indicator. Analyst 135(11):2960–2964. doi:10.1039/c0an00359j
Ai L, Zhang C, Liao F, Wang Y, Li M, Meng L, Jiang J (2011) Removal of methylene blue from aqueous solution with magnetite loaded multi-wall carbon nanotube: kinetic, isotherm and mechanism analysis. J Hazard Mater 198:282–290. doi:10.1016/j.jhazmat.2011.10.041
Li H, Zhao Y, Chen Z, Xu D (2017) Silver enhanced ratiometric nanosensor based on two adjustable fluorescence resonance energy transfer modes for quantitative protein sensing. Biosens Bioelectron 87:428–432. doi:10.1016/j.bios.2016.08.075
Qiu Z, Shu J, He Y, Lin Z, Zhang K, Lv S, Tang D (2017) CdTe/CdSe quantum dot-based fluorescent aptasensor with hemin/G-quadruplex DNzyme for sensitive detection of lysozyme using rolling circle amplification and strand hybridization. Biosens Bioelectron 87:18–24. doi:10.1016/j.bios.2016.08.003
Xu Y, Hun X, Liu F, Wen X, Luo X (2015) Aptamer biosensor for dopamine based on a gold electrode modified with carbon nanoparticles and thionine labeled gold nanoparticles as probe. Microchim Acta 182(9–10):1797–1802. doi:10.1007/s00604-015-1509-5
Song Q, Peng M, Wang L, He D, Ouyang J (2016) A fluorescent aptasensor for amplified label-free detection of adenosine triphosphate based on core-shell Ag@SiO < inf > 2</inf > nanoparticles. Biosens Bioelectron 77:237–241. doi:10.1016/j.bios.2015.09.008
Dai S, Wu S, Duan N, Wang Z (2016) A luminescence resonance energy transfer based aptasensor for the mycotoxin ochratoxin a using upconversion nanoparticles and gold nanorods. Microchim Acta 183(6):1909–1916. doi:10.1007/s00604-016-1820-9
Mahdi M, Mansour B, Afshin M (2016) Competitive immunoassay for ochratoxin a based on FRET from quantum dot-labeled antibody to rhodamine-coated magnetic silica nanoparticles. Microchim Acta 183(12):3093–3099. doi:10.1007/s00604-016-1951-z
Yu FY, Chi TF, Liu BH, Su CC (2005) Development of a sensitive enzyme-linked immunosorbent assay for the determination of ochratoxin a. J Agric Food Chem 53(17):6947–6953. doi:10.1021/jf0513922
Liu XP, Deng YJ, Jin XY, Chen LG, Jiang JH, Shen GL, Yu RQ (2009) Ultrasensitive electrochemical immunosensor for ochratoxin a using gold colloid-mediated hapten immobilization. Anal Biochem 389(1):63–68. doi:10.1016/j.ab.2009.03.019
Majdinasab M, Sheikh-Zeinoddin M, Soleimanian-Zad S, Li P, Zhang Q, Li X, Tang X, Li J (2015) A reliable and sensitive time-resolved fluorescent immunochromatographic assay (TRFICA) for ochratoxin a in agro-products. Food Control 47:126–134. doi:10.1016/j.foodcont.2014.06.044
Taghdisi SM, Danesh NM, Beheshti HR, Ramezani M, Abnous K (2016) A novel fluorescent aptasensor based on gold and silica nanoparticles for the ultrasensitive detection of ochratoxin a. Nanoscale 8(6):3439–3446. doi:10.1039/c5nr08234j
Yang X, Qian J, Jiang L, Yan Y, Wang K, Liu Q, Wang K (2014) Ultrasensitive electrochemical aptasensor for ochratoxin a based on two-level cascaded signal amplification strategy. Bioelectrochemistry 96:7–13
Acknowledgement
Financial support of this study was provided by Mashhad University of Medical Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Additional information
Khalil Abnous and Noor Mohammad Danesh contributed equally to the work.
Rights and permissions
About this article
Cite this article
Abnous, K., Danesh, N.M., Alibolandi, M. et al. Amperometric aptasensor for ochratoxin A based on the use of a gold electrode modified with aptamer, complementary DNA, SWCNTs and the redox marker Methylene Blue. Microchim Acta 184, 1151–1159 (2017). https://doi.org/10.1007/s00604-017-2113-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-017-2113-7