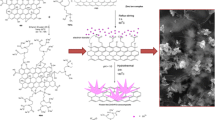
Abstract
We describe the preparation of a nanohybrid consisting of nitrogen doped reduced graphene oxide and CuS nanoparticles (N-rGO/CuS) by in-situ microwave irradiation at weight ratios of 25/75, 50/50, and 75/25. The resulting nanohybrids were characterized by X-ray diffraction, X-ray photoelectron spectroscopy, FTIR, spectroscopy, scanning electron and transmission electron microscopy, electrochemically by cyclic voltammetry and electrochemical impedance analysis. It is shown that the CuS nanoparticles are evenly decorated onto the N-rGO surface. The nanohybrids was placed on glassy carbon electrode (GCE) where they showed electro-reductive activity towards picric acid, typically at working voltages between −0.2 and −0.8 V (vs. SCE). Effects of pH value and scan rate were evaluated, and it is shown that two electrons are involved in electro-reduction. The detection limits of the GCE modified with various N-rGO/CuS hybrids (with 25/75, 50/50, and 75/25 wt%) are 6.2, 3.2, and 0.069 μM respectively. The method demonstrates its applicability in sensing of picric acid with good reproducibility.

Nitrogen doped reduced graphene oxide nanohybrids was synthesized for the detection of picric acid. A straightforward and preconcentration free analysis of picric acid was successfully demonstrated at nanomolar levels using the nanohybrids.








Similar content being viewed by others
References
Wang X, Sun G, Routh P, Kim D-H, Huang W, Chen P (2014) Heteroatom-doped graphene materials: syntheses, properties and applications. Chem Soc Rev 43(20):7067–7098. doi:10.1039/C4CS00141A
Duan X, Ao Z, Sun H, Indrawirawan S, Wang Y, Kang J, Liang F, Zhu ZH, Wang S (2015) Nitrogen-doped graphene for generation and evolution of reactive radicals by metal-free catalysis. ACS Appl Mater Interfaces 7(7):4169–4178. doi:10.1021/am508416n
Shang L, Zeng B, Zhao F (2015) Fabrication of novel nitrogen-doped graphene–hollow AuPd nanoparticle hybrid films for the highly efficient Electrocatalytic reduction of H2O2. ACS Appl Mater Interfaces 7(1):122–128. doi:10.1021/am507149y
Wang R, Xu C, Sun J, Gao L, Yao H (2014) Solvothermal-induced 3D macroscopic SnO2/nitrogen-doped graphene aerogels for high capacity and long-life lithium storage. ACS Appl Mater Interfaces 6(5):3427–3436. doi:10.1021/am405557c
Guo H-L, Peng S, Xu J-H, Zhao Y-Q, Kang X (2014) Highly stable pyridinic nitrogen doped graphene modified electrode in simultaneous determination of hydroquinone and catechol. Sensors Actuators B Chem 193:623–629. doi:10.1016/j.snb.2013.12.018
Gai P, Zhang H, Zhang Y, Liu W, Zhu G, Zhang X, Chen J (2013) Simultaneous electrochemical detection of ascorbic acid, dopamine and uric acid based on nitrogen doped porous carbon nanopolyhedra. Journal of Materials Chemistry B 1(21):2742–2749. doi:10.1039/C3TB20215A
Wu L, Lei W, Han Z, Zhang Y, Xia M, Hao Q (2015) A novel non-enzyme amperometric platform based on poly(3-methylthiophene)/nitrogen doped graphene modified electrode for determination of trace amounts of pesticide phoxim. Sensors Actuators B Chem 206:495–501. doi:10.1016/j.snb.2014.09.098
Xue X, Wei Q, Wu D, Li H, Zhang Y, Feng R, Du B (2014) Determination of methyl parathion by a molecularly imprinted sensor based on nitrogen doped graphene sheets. Electrochim Acta 116:366–371. doi:10.1016/j.electacta.2013.11.075
Dai H, Zhang S, Lin Y, Ma Y, Gong L, Xu G, Fu M, Li X, Chen G (2014) Amplified electrochemiluminescence of lucigenin triggered by electrochemically reduced graphene oxide and its sensitive detection of bisphenol a. Anal Methods 6:4746–4753. doi:10.1039/c4ay00750f
Giribabu K, Suresh R, Manigandan R, Praveen Kumar S, Muthamizh S, Munusamy S, Narayanan V (2014) Preparation of nitrogen-doped reduced graphene oxide and its use in a glassy carbon electrode for sensing 4-nitrophenol at nanomolar levels. Microchim Acta 181(15):1863–1870. doi:10.1007/s00604-014-1251-4
Ahmed GHG, Laíño RB, Calzón JAG, García MED (2015) Highly fluorescent carbon dots as nanoprobes for sensitive and selective determination of 4-nitrophenol in surface waters. Microchim Acta 182(1–2):51–59
Deng P, Xu Z, Li J (2014) Simultaneous voltammetric determination of 2-nitrophenol and 4-nitrophenol based on an acetylene black paste electrode modified with a graphene-chitosan composite. Microchim Acta 181(9–10):1077–1084
Yang X, Niu C-G, Shen G-L, Yu R-Q (2001) Picric acid sensitive optode based on a fluorescence carrier covalently bound to membrane. Analyst 126(3):349–352. doi:10.1039/B009002F
Pamme N, Steinbach K, Ensinger WJ, Schmidt TC (2002) Analysis of polynitrophenols and hexyl by liquid chromatography–mass spectrometry using atmospheric pressure ionisation methods and a volatile ion-pairing reagent. J Chromatogr A 943(1):47–54
Junqueira JRC, de Araujo WR, Salles MO, Paixão TRLC (2013) Flow injection analysis of picric acid explosive using a copper electrode as electrochemical detector. Talanta 104:162–168. doi:10.1016/j.talanta.2012.11.036
Jacobsen M, Duwensee H, Wachholz F, Adamovski M, Flechsig G-U (2010) Directly heated bismuth film electrodes based on gold Microwires. Electroanalysis 22(13):1483–1488. doi:10.1002/elan.200900486
Vyskočil V, Navrátil T, Daňhel A, Dědík J, Krejčová Z, Škvorová L, Tvrdíková J, Barek J (2011) Voltammetric determination of selected nitro compounds at a polished silver solid amalgam composite electrode. Electroanalysis 23(1):129–139. doi:10.1002/elan.201000428
del Mar C-RM, Naranjo-Rodríguez I, Palacios-Santander JM, Cubillana-Aguilera LM, Hidalgo-Hidalgo-de-Cisneros JL (2005) Study of the responses of a sonogel-carbon electrode towards phenolic compounds. Electroanalysis 17(9):806–814. doi:10.1002/elan.200403157
Hummers WS, Offeman RE (1958) Preparation of graphitic oxide. J Am Chem Soc 80(6):1339–1339. doi:10.1021/ja01539a017
Liu L, Zhong H, Bai Z, Zhang T, Fu W, Shi L, Xie H, Deng L, Zou B (2013) Controllable transformation from rhombohedral Cu1.8S nanocrystals to hexagonal CuS clusters: phase- and composition-dependent Plasmonic properties. Chem Mater 25(23):4828–4834. doi:10.1021/cm403420u
Lee G-J, Anandan S, Masten SJ, Wu JJ (2014) Sonochemical synthesis of hollow copper doped zinc sulfide nanostructures: optical and catalytic properties for visible light assisted Photosplitting of water. Ind Eng Chem Res 53(21):8766–8772. doi:10.1021/ie500663n
Yu J, Ran J (2011) Facile preparation and enhanced photocatalytic H2-production activity of Cu(OH)2 cluster modified TiO2. Energy & Environmental Science 4(4):1364–1371. doi:10.1039/C0EE00729C
Jama C, Rousseau V, Dessaux O, Goudmand P (1997) Carbon nitride CNx film deposition assisted by IR laser ablation in a cold remote nitrogen plasma. Thin Solid Films 302(1–2):58–65. doi:10.1016/S0040-6090(96)09541-7
He D, Jiang Y, Lv H, Pan M, Mu S (2013) Nitrogen-doped reduced graphene oxide supports for noble metal catalysts with greatly enhanced activity and stability. Appl Catal B Environ 132–133:379–388. doi:10.1016/j.apcatb.2012.12.005
Arrigo R, Havecker M, Schlogl R, Su DS (2008) Dynamic surface rearrangement and thermal stability of nitrogen functional groups on carbon nanotubes. Chem Commun 40:4891–4893. doi:10.1039/B812769G
Yeryukov NA, Milekhin AG, Sveshnikova LL, Duda TA, Pokrovsky LD, Gutakovskii AK, Batsanov SA, Rodyakina EE, Latyshev AV, Zahn DRT (2014) Synthesis and characterization of CuxS (x = 1–2) nanocrystals formed by the Langmuir–Blodgett technique. J Phys Chem C 118(40):23409–23414. doi:10.1021/jp507355t
Ishii M, Shibata K, Nozaki H (1993) Anion distributions and phase transitions in CuS1-xSex(x = 0-1) studied by Raman spectroscopy. J Solid State Chem 105(2):504–511. doi:10.1006/jssc.1993.1242
Shao G, Lu Y, Wu F, Yang C, Zeng F, Wu Q (2012) Graphene oxide: the mechanisms of oxidation and exfoliation. J Mater Sci 47(10):4400–4409. doi:10.1007/s10853-012-6294-5
Tu W, Liu H (2000) Continuous synthesis of colloidal metal nanoclusters by microwave irradiation. Chem Mater 12(2):564–567. doi:10.1021/cm990637l
Lamache M, Bauer D (1979) Anodic oxidation of cuprous sulfide and the preparation of nonstoichiometric copper sulfide. Anal Chem 51(8):1320–1322. doi:10.1021/ac50044a045
de Tacconi NR, Rajeshwar K, Lezna RO (1996) Study of copper sulfide film formation by voltammetry combined with electrochemical quartz crystal Microgravimetry/Coulometry and optical spectroscopy. J Phys Chem 100(46):18234–18239. doi:10.1021/jp962125p
Brownson DAC, Gorbachev RV, Haigh SJ, Banks CE (2012) CVD graphene vs. highly ordered pyrolytic graphite for use in electroanalytical sensing. Analyst 137(4):833–839. doi:10.1039/C2AN16049H
Pontié M, Thouand G, De Nardi F, Tapsoba I, Lherbette S (2011) Antipassivating electrochemical process of glassy carbon electrode (GCE) dedicated to the oxidation of nitrophenol compounds. Electroanalysis 23(7):1579–1584. doi:10.1002/elan.201100082
Gong L, Yan H, Dai H, Zhang S, Li Y, Zhang Q, Lin Y, Hong Z (2016) Highly sensitive and stabilized sensing of 6-benzylaminopurine based on NiCo2O4 nanosuperstructures. RSC Adv 6:4758–4763. doi:10.1039/C5RA23343G
Huang J, Wang L, Shi C, Dai Y, Gu C, Liu J (2014) Selective detection of picric acid using functionalized reduced graphene oxide sensor device. Sensors Actuators B Chem 196:567–573. doi:10.1016/j.snb.2014.02.050
Acknowledgments
K. Giribabu thanks the Department of Science and Technology (DST), Government of India for financial assistance in the form of INSPIRE fellowship (Inspire Fellow no: 10226) under the AORC scheme. The authors gratefully acknowledge the financial support received from the National Research Foundation of Korea (NRF) grant by the Korea government Ministry of Education, Science and Technology (MEST) (NRF-2014R1A5A1009799). The authors also acknowledge the National Centre for Nanoscience and Nanotechnology (NCNSNT), University of Madras for conducting XPS, FESEM and HRTEM analyses.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Compliance with ethical standards
The author(s) declare that they have no competing interests.
Additional information
Krishnan Giribabu and Seo Yeong Oh contributed equally to this work
Electronic Supplementary Material
ESM 1
(DOCX 518 kb)
Rights and permissions
About this article
Cite this article
Giribabu, K., Oh, S.Y., Suresh, R. et al. Sensing of picric acid with a glassy carbon electrode modified with CuS nanoparticles deposited on nitrogen-doped reduced graphene oxide. Microchim Acta 183, 2421–2430 (2016). https://doi.org/10.1007/s00604-016-1883-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-016-1883-7