Abstract
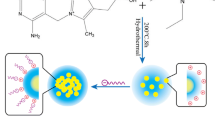
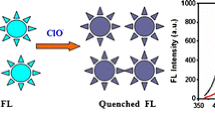
We report on the synthesis of carbon dots (C-dots) by thermal carbonization of a mixture of ethyleneglycol bis-(2-aminoethyl ether)-N,N,N’,N’-tetraacetic acid (EGTA) and tris(hydroxymethyl)aminomethane (Tris). The resulting C-dots were characterized by X-ray diffraction, proton and carbon nuclear magnetic resonance, FTIR and fluorescence spectroscopy, and high-resolution TEM. The data reveal that the C-dots are mainly capped with hydroxy and carbonyl groups and are highly fluorescent with an emission peak that shifts from 427 to 438 nm if the excitation wavelength is increased from 310 to 360–370 nm. Fluorescence is quenched by 4-nitrophenol (4-NP), and this effect was exploited to design a simple and rapid protocol for the determination of 4-NP. The detection limit is 28 nM and the linear range extends from 0.1 to 50 μM. The method was successfully applied to the determination of 4-NP in spiked river and sea waters.

Contact quenching produced through the formation of a Meisenheimer complex with negative charge delocalized over the cyclohexadienine ring and the nitro group and positive charge distributed over an iminium group.






Similar content being viewed by others
References
Baker SN, Baker GA (2010) Luminescent carbon nanodots: emergent nanolights. Angew Chem Int Ed 49:6726
Esteves da Silva JCG, Gonҫalves HMR (2011) Analytical and bioanalytical applications of carbon dots. TrAC Trends Anal Chem 30:1327
Li H, Kang Z, Liu Y, Lee ST (2012) Carbon nanodots: synthesis, properties and applications. J Mater Chem 22:24230
Demchenko AP, Dekaliuk MO (2013) Novel fluorescent carbonic nanomaterials for sensing and imaging. Methods Appl Fluoresc 1:042001
Yang Z, Li Z, Xu M, Ma Y, Zhang J, Su Y, Gao F, Wei H, Zhang L (2013) Controllable synthesis of fluorescent carbon dots and their detection application as nanoprobes. Nano Micro Lett 5:247
Liu LQ, Li YF, Zhan L, Liu Y, Huang CZ (2011) One-step synthesis of fluorescent hydroxy coated carbon dots wiyh hydrothermal reaction and its application to optical sensing of metal ions. Sci China Ser B Chem 54:1342
Zhou L, Lin YH, Huang ZZ, Ren JS, Qu XG (2012) Carbon nanodots as fluorescence probes for rapid, sensitive, and label-free detection of Hg2+ and biothiols in complex matrices. Chem Commun 48:1147
Zhu AW, Qu Q, Shao XL, Kong B, Tian Y (2012) Carbon-dot-based dual-emission nanohybrid produces a ratiometric fluorescent sensor for in vivo imaging of cellular copper ions. Angew Chem Int Ed 51:7185
Dong YQ, Wang RX, Li H, Shao JW, Chi YW, Lin XM, Chen GN (2012) Polyamine-functionalized carbon quantum dots for chemical sensing. Carbon 50:2810
Lai T, Zheng E, Chen L, Wang X, Kong L, You C, Ruan Y, Weng X (2013) Hybrid carbon source for producing nitrogen-doped polymer nanodots: one-pot hydrothermal synthesis, fluorescence enhancement and highly selective detection of Fe(III). Nanoscale 5:8015
Bai WJ, Zheng HZ, Long YJ, Mao XJ, Gao M, Zhang L (2011) A carbon dots-based fluorescence turn-on method for DNA determination. Anal Sci 27:243
Wang CI, Wu WC, Periasamy AP, Chang HT (2014) Electrochemical synthesis of photoluminescent carbon nanodots from glycine for highly sensitive detection of hemoglobin. Green Chem. doi:10.1039/C3GC42325E
Wei J, Quiang L, Ren J, Ren X, Meng X, Tang F (2014) Fluorescence turn-off detection of hydrogen peroxide and glucose directly using carbon nanodots as probes. Anal Methods. doi:10.1039/C3AY41837E
Baruah U, Gogoi N, Majumdar G, Chowdhury D (2013) Capped fluorescent carbon dots for detection of hemin: role of number of –OH groups of capping agent in fluorescence quenching. Sci World J, Article ID 529159, 9 pages. doi:10.1155/2013/529159
Qu KG, Wang JS, Ren JS, Qu XG (2013) Carbon dots prepared by hydrothermal treatment of dopamine as an effective fluorescent sensing platform for the label-free detection. Chem Eur J 19:7243
Mononitrophenols. Concise International Chemical Assessment Document. (CICADs) (2000), World Health Organization, Geneva
Lin F, Pei D, He W, Huang Z, Huang Y, Guo X (2012) Electron transfer quenching by nitroxide radicals of the fluorescence of carbon dots. J Mater Chem 22:11801
Wang X, Cao L, Lu F, Meziani MJ, Li H, Qi G, Zhou B, Harruff BA, Kermarrec F, Sun YP (2009) Photoinduced electron transfers with carbon dots. Chem Commun 25:3774
Niu Q, Gao K, Lin Z, Wu W (2013) Amine-capped carbon dots as a nanosensor for sensitive and selective detection of picric acid in aqueous solution via electrostatic interaction. Anal Methods 5:6228
Lakowicz JR (1999) Principles of fluorescence spectroscopy, 2nd edn. Kluwer Academic/Plenum, New York
Smilgies DM (2009) Scherrer grain-size analysis adapted to grazing incidence scattering with area detectors. J Appl Crystallogr 42:1030
Short MA, Walker PL (1965) Measurement of interlayer spacing and crystal sizes in turbostatic carbons. Carbon 1:3
Sadezky A, Muckenhuber H, Grothe H, Niessner R, Pöschl U (2005) Raman microspectroscopy of soot and related carbonaceous materials: spectral analysis and structural information. Carbon 43:1731
AIST: RIO-DB. Spectral database for organic compounds, SDBS: http://riodb01.ibase.aist.go.jp/sdbs/cgi-bin/cre_index.cgi?lang=eng
Tang L, Ji R, Cao X, Lin J, Jiang H, Li X, Teng KS, Luk CM, Zeng S, Hao J, Lau SP (2012) Deep ultraviolet photoluminescence of water-soluble self-passivated graphene quantum dots. ACS Nano 6:5102
Liu XJ, Guo ML, Huang J, Yin XY (2013) Improved fluorescence of carbon dots prepared from bagasse under alkaline hydrothermal conditions. Bioresources 8:2537
De B, Karak N (2013) A green and facile approach for the synthesis of water soluble fluorescent carbon dots from banana juice. RSC Adv 3:8286
Mao LH, Tang WQ, Deng ZY, Liu SS, Wang CF, Chen S (2014) Facile access to white fluorescent carbon dots toward light emitting devices. Ind Eng Chem Res 53:6417
Qiao ZA, Wang Y, Gao Y, Li H, Dai T, Liu Y, Huo Q (2010) Chem Commun 46:8812
US Environmental Protection Agency, Nitrophenols, Ambient Water Quality Criteria. Criteria and Standards Division Office of Water Planning and Standards (1980), Washington, DC
Al-Kaysi RO, Creed D, Valente EJ (2004) Meisenheimer complex from picric acid and diisopropylcarbodiimide. J Chem Crystallogr 34:685
Giribabu K, Suresh R, Manigandan R, Munusamy S, Kumar SP, Muthamizh S, Narayanan V (2013) Nanomolar determination of 4-nitrophenol based on a poly(methylene blue)-modified glassy carbon electrode. Analyst 138:5811
Li J, Kuang D, Feng Y, Zhang F, Xu Z, Liu M (2012) A graphene oxide-based electrochemical sensor for sensitive determination of 4-nitrophenol. J Hazard Mater 201–202:250
Yin H, Zhou Y, Ai S, Liu X, Zhu L, Lu L (2010) Electrochemical oxidative determination of 4-nitrophenol based on a glassy carbon electrode modified with a hydroxyapatite nanopowder. Microchim Acta 169:87
Gu Y, Zhang Y, Zhang F, Wei J, Wang C, Du Y, Ye W (2010) Electrochim Acta 56:953
Tapsoba I, Bourhis S, Feng T, Pontié M (2009) Sensitive and selective electrochemical analysis of methyl-parathion (MPT) and 4-nitrophenol (PNP) by a new type of p-NiTSPc/pPPD coated carbon fiber microelectrode (CFME). Electroanalysis 21:1167
Huang W, Yang C, Zhan S (2003) Simultaneous determination of 2-nitrophenol and 4-nitrophenol based on the multi-wall carbon nanotubes nafion-modified electrode. Anal Bioanal Chem 375:703
Du F, Zeng F, Ming Y, Wu S (2013) Carbon dots-based fluorescent probes for sensitive and selective detection of iodide. Microchim Acta 180:453
Yazid SNA, Chia SF, Pang SC, Ng SM (2013) Detection of Sn(II) ions via quenching of the fluorescence of carbon dots. Microchim Acta 180:137
Acknowledgments
Authors gratefully acknowledge financial support from the Science and Innovation Spanish Ministry (Proj # MAT2012-099). Also, G.H.Gaber Ahmed thanks an Erasmus Mundus Medastar grant.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 304 kb)
Rights and permissions
About this article
Cite this article
Ahmed, G.H.G., Laíño, R.B., Calzón, J.A.G. et al. Highly fluorescent carbon dots as nanoprobes for sensitive and selective determination of 4-nitrophenol in surface waters. Microchim Acta 182, 51–59 (2015). https://doi.org/10.1007/s00604-014-1302-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-014-1302-x