Abstract
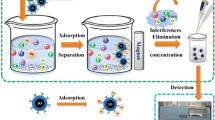
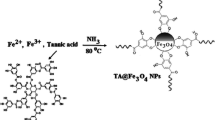
We describe a magnetic metal-organic framework for preconcentration of Hg(II). The material is obtained from magnetite (Fe3O4) nanoparticles that were modified with 4-(5)-imidazoledithiocarboxylic acid and then reacted with trimesic acid and Cu(II) acetate to form the metal-organic framework capable of extracting Hg(II). The sorption time, amount of the magnetic nanocomposite, and pH value of the sample were selected as the main affecting factors in sorption, and central composite design and response surface methodology were applied to optimize these parameters. Following sorption of Hg(II), the sorbent is removed by a magnet, Hg(II) is eluted with a solution of thiourea and then quantified by cold vapor AAS. The type, volume and concentration of the eluent, and the elution time were selected for the optimization of the elution. The results showed the sorption process to obey the Langmuir model. The maximum monolayer capacity is as high as 254 mg g−1, and the Langmuir constant is 0.330 L mg−1. The findings can be well described by pseudo second-order kinetics. High sorption capacity means that one needs less sorbent. Under the optimal conditions, the limit of detection and limit of quantification for Hg(II) were 10 ng L−1 and 40 ng L−1, respectively and the relative standard deviations are <8.3 %. The nanocomposite was successfully applied to the rapid extraction of trace amounts of mercury ions from fish and canned tuna samples.

The article describes the synthesis and application of a novel magnetic metal-organic framework for preconcentration of Hg(II) ion, and its determination by cold vapor atomic absorption spectrometry. The LOD is as low as 10 ng L−1.


Similar content being viewed by others
References
Kuban P, Houserova P, Kuban P, Hauser PC, Kuban V (2007) Sensitive capillary electrophoretic determination of mercury species with amperometric detection at a copper electrode after cation exchange preconcentration. J Sep Sci 30:1070–1076
Cossa D, Sanjuan J, Cloud J, Stockwell PB, Toms WT (1995) Automated technique for mercury determination at sub-nanogram per litre levels in natural waters. J Anal Spectrom 10:287–291
Dakova I, Karadjova I, Georgieva V, Georgiev G (2009) Ion-imprinted polymethacrylic microbeads as new sorbent for preconcentration and speciation of mercury. Talanta 78:523–529
Zhai Y, Duan S, He Q, Yang X, Han Q (2010) Solid phase extraction and preconcentration of trace mercury(II) from aqueous solution using magnetic nanoparticles doped with 1,5-diphenylcarbazide. Microchim Acta 169:353–360
Tuzen M, Soylak M (2005) Mercury contamination in mushroom samples from Tokat, Turkey. Bull Environ Contam Toxicol 74:968–972
Baghdadi M, Shemirani F (2008) Cold-induced aggregation microextraction: A novel sample preparation technique based on ionic liquids. Anal Chim Acta 613:56–63
Bryce DW, Izquierdo A, Luque de Castro MD (1996) Continuous microwave assisted pervaporation/atomic fluorescence detection: An approach for speciation in solid samples. Anal Chim Acta 324:69–75
de Wuilloud JCA, Wuilloud RG, Silva MF, Olsina RA, Martinez LD (2002) Sensitive determination of mercury in tap water by cloud point extraction pre-concentration and flow injection-cold vapor-inductively coupled plasma optical emission spectrometry. Spectrochim Acta Part B 57:365–374
Chen J, Chen H, Jin X, Chen H (2009) Determination of Ultra-trace amount methyl-, phenyl- and inorganic mercury in environmental and biological samples by liquid chromatography with inductively coupled plasma mass spectrometry after cloud point extraction preconcentration. Talanta 77:1381–1387
Karadjova I, Arpadjan S, Cvetkovic J, Stafilov T (2004) Sensitive method for trace determination of mercury in wines using electrothermal atomic absorption spectrometry. Microchim Acta 147:39–43
Leyva D, Esteˇıvez J, Montero A, Pupo I (2007) Sub-ppm determination of Hg and Cr in water: Cr speciation. X-Ray Spectrom 36:355–360
Hosseini-Bandegharaei A, Hosseini MS, Jalalabadi Y, Sarwghadi M, Nedaie M, Taherian A, Ghaznavi A, Eftekhari A (2011) Removal of Hg(II) from aqueous solutions using a novel impregnated resin containing 1-(2-thiazolylazo)-2-naphthol (TAN). Chem Eng J 168:1163–1173
Horvat M, Liang L, Bloom NS (1993) Comparison of distillation with other current isolation methods for the determination of methyl mercury compounds in low level environmental samples, part II: Water. Anal Chim Acta 282:153–168
Bic N, Sungur S, Gazi M, Tan N (2003) Selective liquid-liquid extraction of mercuric ions by octyl methane sulfonamide. Sep Sci Technol 38:201–217
Tuzen M, Saygi KO, Soylak M (2008) Solid phase extraction of heavy metal ions in environmental samples on multiwalled carbon nanotubes. J Hazard Mater 152:632–639
Tuzen M, Karaman I, Citak D, Soylak M (2009) Mercury (II) and methyl mercury determinations in water and fish samples by using solid phase extraction and cold vapour atomic absorption spectrometry combination. Food Chem Toxicol 47:1648–1652
Tuzen M, Uluozlu OD, Karaman I, Soylak M (2009) Mercury (II) and methyl mercury speciation on streptococcus pyogenes loaded dowex optipore SD-2. J Hazard Mater 169(345):350
Faraji M, Yamini Y, Rezaee M (2010) Extraction of trace amounts of mercury with sodium dodecyle sulphate-coated magnetite nanoparticles and its determination by flow injection inductively coupled plasma-optical emission spectrometry. Talanta 81:831–836
Pourreza N, Ghanemi K (2009) Determination of mercury in water and fish samples by cold vapor atomic absorption spectrometry after solid phase extraction on agar modified with 2-mercaptobenzimidazole. J Hazard Mater 161:982–987
Sohrabi MR (2014) Preconcentration of mercury(II) using a thiol-functionalized metal-organic framework nanocomposite as a sorbent. Microchim Acta 181:435–444
Ghorbani-Kalhor E, Hosseinzadeh-Khanmiri R, Abolhasani J, Babazadehd M, Hassanpour A (2015) Determination of mercury(II) ions in seafood samples after extraction and preconcentration by a novel functionalized magnetic metal-organic framework nanocomposite. J Sep Sci 38:1179–1186
Chae HK, Siberio-Perez DY, Kim J, Go Y, Eddaoudi M, Matzger AJ, O’Keeffe M, Yaghi OM (2004) A route to high surface area, porosity and inclusion of large molecules in crystals. Nature 427:523–527
Shekhah O, Liu J, Fischer RA, Wöll C (2011) MOF thin films: existing and future applications. Chem Soc Rev 40:1081–1106
Barreto AS, da Silva RL, dos Santos Silva SCG, Rodrigues MO, de Simone CA, de Sá GF, Júnior SA, Navickiene S, de Mesquita ME (2010) Potential of a metal-organic framework as a new material for solid-phase extraction of pesticides from lettuce (Lactuca sativa), with analysis by gas chromatography-mass spectrometry. J Sep Sci 33:3811–3816
Harvey SD, Eckberg AD, Thallapally PK (2011) Evaluation of copper-1, 3, 5-benzenetricarboxylate metal-organic framework (Cu-MOF) as a selective sorbent for Lewis-base analytes. J Sep Sci 34:2418–2426. Chicago.
Ke F, Yuan Y-P, Qiu L-G, Shen Y-H, Xie A-J, Zhu J-F, Tianc X-Y, Zhang L-D (2011) Facile fabrication of magnetic metal-organic framework nanocomposites for potential targeted drug delivery. J Mater Chem 21:3843–3848
Taghizadeh M, Asgharinezhad AA, Pooladi M, Barzin M, Abbaszadeh A, Tadjarodi A (2013) A novel magnetic metal organic framework nanocomposite for extraction and preconcentration of heavy metal ions, and its optimization via experimental design methodology. Microchim Acta 180:1073–1084
Yilmaz AB (2003) Levels of heavy metals (Fe, Cu, Ni, Cr, Pb, and Zn) in tissue of Mugil cephalus and Trachurus mediterraneus from Iskenderun Bay. Turkey Environ Res 92:277–281
Asgharinezhad AA, Ebrahimzadeh H, Rezvani M, Shekari N, Loni M (2014) A novel 4-(2 pyridylazo) resorcinol functionalized magnetic nanosorbent for selective extraction of Cu(II) and Pb(II) ions from food and water samples. Food Addit Contam 31:1196–1204
Hartmann M, Kunz S, Himsl D, Tangermann O, Ernst S, Wagener A (2008) Adsorptive separation of isobutene and isobutane on Cu3(BTC)2. Langmuir 24:8634–8642
Abolhasani J, Khanmiri RH, Ghorbani-Kalhor E, Hassanpour A, Asgharinezhad AA, Shekari N, Fathi A (2015) An Fe3O4@SiO2@polypyrrole magnetic nanocomposite for the extraction and preconcentration of Cd(II) and Ni(II). Anal Methods 7:313–320
Wang Y, Xie J, Wu Y, Hu X (2014) A magnetic metal-organic framework as a new sorbent for solid-phase extraction of copper (II), and its determination by electrothermal AAS. Microchim Acta 181:949–956
Mohan D, Gupta VK, Srivastava SK, Chander S (2001) Kinetics of mercury adsorption from wastewater using activated carbon derived from fertilizer waste. Colloid Surf A 177:169–181
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests
Electronic supplementary material
ESM 1
(DOCX 289 kb)
Rights and permissions
About this article
Cite this article
Tadjarodi, A., Abbaszadeh, A. A magnetic nanocomposite prepared from chelator-modified magnetite (Fe3O4) and HKUST-1 (MOF-199) for separation and preconcentration of mercury(II). Microchim Acta 183, 1391–1399 (2016). https://doi.org/10.1007/s00604-016-1770-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-016-1770-2