Abstract
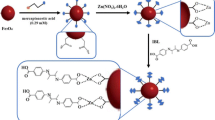
Magnetite (Fe3O4) nanoparticles were coated with tannic acid to give nanoparticles (NPs) of the type Fe3O4@TA and are shown to be a viable sorbent for preconcentration of Cd2+, Co2+ and Cr3+. The size, morphology, composition, and properties of the Fe3O4@TA NPs were characterized by field emission scanning electron microscopy, energy-dispersive X-ray analysis, vibrating sample magnetometery and FTIR. They were applied to the solid-phase extraction of the metal ions from environmental water samples prior to their quantitation by flow injection inductively coupled plasma-optical emission spectrometry. The effects of sample solution, extraction and desorption times, kind of eluent and quantity of sorbent were optimized. The calibration plots are linear in the concentration ranges from 0.5 to 100 μg L−1 (for both Cd and Co) and from 0.2 to 100 μg L−1 (for Cr). The limits of detection are between 0.1 and 0.2 μg L−1. The intra-day relative standard deviations based on four replicates are in the range of 6.1 to 7.1 %. The method was successfully applied to the determination of the three metal ions in (spiked) tap water, mineral water, and river water. Recoveries varied in the range from 90 to 109 %, this confirming the good performance of the method.

Tannic acid-coated Fe3O4 nanoparticles (TA@ Fe3O4 NPs) have been successfully synthesized. The NPs were applied as an efficient sorbent for extraction and pre-concentration of Cd2+, Co2+ and Cr3+ from environmental water samples.



Similar content being viewed by others
References
Fu F, Wang Q (2011) Removal of heavy metal ions from wastewaters: a review. J Environ Manag 92:407. doi:10.1016/j.jenvman.2010.11.011
Manzoori JL, Abdolmohammad-Zadeh H, Amjadi M (2007) Simplified cloud point extraction for the preconcentration of ultra-trace amounts of gold prior to determination by electrothermal atomic absorption spectrometry. Microchim Acta 159:71. doi:10.1007/s00604-0727-2
Cui Y, Chang X, Zhu X, Luo H, Hu Z, Zou X, He Q (2007) Chemically modified silica gel with p-dimethylaminobenzaldehyde for selective solid-phase extraction and preconcentration of Cr(III), Cu(II), Ni(II), Pb(II) and Zn(II) by ICP-OES. Microchem J 87:20. doi:10.1016/j.microc.2007.04.004
Li J, Zhong L-F, Tu X-L, Liang X-R, Xu J-F (2010) Determination of rhenium content in molybdenite by ICP–MS after separation of the major matrix by solvent extraction with N-benzoyl-N-phenylhydroxalamine. Talanta 81:954. doi:10.1016/j.talanta.2010.01.043
Praveen RS, Daniel S, Prasada Rao T (2005) Solid phase extraction preconcentration of cobalt and nickel with 5,7-dichloroquinone-8-ol embedded styrene–ethylene glycol dimethacrylate polymer particles and determination by flame atomic absorption spectrometry (FAAS). Talanta 66:513. doi:10.1016/j.talanta.2004.11.026
Yamini Y, Faraji M, Adeli M (2015) Magnetic silica nanomaterials for solid-phase extraction combined with dispersive liquid-liquid microextraction of ultra-trace quantities of plasticizers. Microchim Acta 182:1491. doi:10.1007/s00604-015-1474-z
Banda R, Jeon H, Lee M (2012) Solvent extraction separation of Pr and Nd from chloride solution containing La using cyanex 272 and its mixture with other extractants. Sep Purif Technol 98:481. doi:10.1016/j.seppur.2012.08.015
NumanBulut V, Duran C, Gundogdu A, Soylak M, Yildirim N, Elci L (2008) A new approach to separation and pre-concentration of some trace metals with co-precipitation method using a triazole. Talanta 76:469. doi:10.1016/j.talanta.2008.03.040
Ojeda CB, Rojas FS (2012) Separation and preconcentration by cloud point extraction procedures for determination of ions: recent trends and applications. Microchim Acta1 77:1. doi:10.1007/s00604-011-0717-x
Badawy NA, El-Baya AA, Abdel-Aal AY, Garamon SE (2009) Chromatographic separations and recovery of lead ions from a synthetic binary mixtures of some heavy metal using cation exchange resin. J Hazard Mater 166:1266. doi:10.1016/j.jhazmat.2008.12.044
Faraji M, Yamini Y, Saleh A, Rezaee M, Ghambarian M, Hassani R (2010) A nanoparticle-based solid-phase extraction procedure followed by flow injection inductively coupled plasma-optical emission spectrometry to determine some heavy metal ions in water samples. Anal Chim Acta 659:172. doi:10.1016/j.aca.2009.11.053
Cui Y, Liu S, Wei K, Liu Y, Hu Z (2015) Magnetic solid-phase extraction of trace-level mercury(II) ions using magnetic core-shell nanoparticles modified with thiourea-derived chelating agents. Microchim Acta 182:1337-. doi:10.1016/j.cej.2013.12.067
Soylak M, Ercan O (2009) Selective separation and preconcentration of copper (II) in environmental samples by the solid phase extraction on multi-walled carbon nanotubes. J Hazard Mater 168:1527. doi:10.1016/j.jhazmat.2009.03.032
Samadi A, Amjadi M (2015) Magnetic Fe3O4@C nanoparticles modified with 1-(2-thiazolylazo)-2-naphthol as a novel solid-phase extraction sorbent for preconcentration of copper (II) Volume. Microchim Acta182:257. doi:10.1016/j.jhazmat.2012.04.001
Zang Z, Li Z, Zhang L, Li R, Hu Z, Chang X, Cui Y (2010) Chemically modified attapulgite with asparagine for selective solid-phase extraction and preconcentration of Fe(III) from environmental samples. Anal Chim Acta 663:213. doi:10.1016/j.aca.2010.01.057
Tahmasebi E, Yamini Y, Moradi M, Esrafili A (2013) Polythiophene-coated Fe3O4 superparamagnetic nanocomposite: synthesis and application as a new sorbent for solid-phase extraction. Anal Chim Acta 770:68. doi:10.1016/j.aca.2013.01.043
Moghanian H, Mobinikhaledi A, Blackman AG, Sarough-Farahani E (2014) Sulfanilic acid-functionalized silica-coated magnetite nanoparticles as an efficient, reusable and magnetically separable catalyst for the solvent-free synthesis of 1-amido- and 1-aminoalkyl-2-naphthols. RSC Adv 4:28176. doi:10.1039/c4ra03676j
Aguilar-Arteaga K, Rodriguez JA, Barrado E (2010) Magnetic solids in analytical chemistry: a review. Anal Chim Acta 674:157. doi:10.1016/j.aca.2010.06.043
Giakisikli G, Anthemidis AN (2013) Magnetic materials as sorbents for metal/metalloid preconcentration and/or separation. A Review Anal Chim Acta 789:1. doi:10.1016/j.chroma.2008.01.047b
Yao N, Chen H, Lin H, Deng C, Zhang X (2008) Enrichment of peptides in serum by C8-functionalized magnetic nanoparticles for direct matrix-assisted laser desorption/ionization time-of-flight mass spectrometry analysis. J Chromatogr A 1185:93. doi:10.1016/j.chroma.2008.01.047
Jiang C, Sun Y, Yu X, Gao Y, Zhang L, Wang Y, Zhang H, Song D (2014) Application of C18-functional magnetic nanoparticles for extraction of aromatic amines from human urine. J Chromatogr B 947–948:49. doi:10.1016/j.jchromb.2013.12.008
Zhang S, Niu H, Cai Y, Shi Y (2010) Barium alginate caged Fe3O4-C18 magnetic nanoparticles for the pre-concentration of polycyclic aromatic hydrocarbons and phthalate esters from environmental water samples. Anal Chim Acta 665:167. doi:10.1016/j.aca.2010.03.026
Ding J, Gao Q, Luo D, Shi Z-G, Feng Y-Q (2010) n-Octadecylphosphonic acid grafted mesoporous magnetic nanoparticle: Preparation, characterization, and application in magnetic solid-phase extraction. J Chromatogr A 1217:7351. doi:10.1016/j.chroma.2010.09.074
Luo C, Tian Z, Yang B, Zhang L, Yan S (2013) Manganese dioxide/iron oxide/acid oxidized multi-walled carbon nanotube magnetic nanocomposite for enhanced hexavalent chromium removal. Chem Eng J 234:256. doi:10.1016/j.cej.2013.08.084
Huang C, Hu B (2008) Silica-coated magnetic nanoparticles modified with γ-mercaptopropyltrimethoxysilane for fast and selective solid phase extraction of trace amounts of Cd, Cu, Hg, and Pb in environmental and biological samples prior to their determination by inductively coupled plasma mass spectrometry. Spectrochim Acta B 63:437. doi:10.1016/j.sab.2007.12.010
Mashhadizadeh MH, Karami Z (2011) Solid phase extraction of trace amounts of Ag, Cd, Cu, and Zn in environmental samples using magnetic nanoparticles coated by 3-(trimethoxysilyl)-1-propantiol and modified with 2-amino-5-mercapto-1,3,4-thiadiazole and their determination by ICP-OES. J Hazard Mater 190:1023. doi:10.1016/j.jhazmat.2011.04.051
Zhang X, Zhang P, Wu Z, Zhang L, Zeng G, Zhou C (2013) Adsorption of methylene blue onto humic acid-coated Fe3O4 nanoparticles. Colloid Surf A 435:85. doi:10.1016/j.colsurfa.2012.12.056
Üçer A, Uyanik A, Aygün ŞF (2006) Adsorption of Cu(II), Cd(II), Zn(II), Mn(II) and Fe(III) ions by tannic acid immobilised activated carbon. Sep Purif Technol 47:113. doi:10.1016/j.seppur.2005.06.012
Slabbert N (1992) Complexation of condensed tannins with metal ions. In: Hemingway RW, Laks PE (eds) Plant polyphenols. Plenum Press, New York, pp. 421–436
Mazur M, Barras A, Kuncser V, Galatanu A, Zaitzev V, Turcheniuk KV, Woisel P, Lyskawa J, Laure W, Siriwardena A, Boukherroub R, Szunerits S (2013) Iron oxide magnetic nanoparticles with versatile surface functions based on dopamine anchors. Nanoscale 5:2692. doi:10.1039/c3nr33506b
Moghanian H, Mobinikhaledi A, Baharangiz Z (2014) Synthesis, characterization and magnetic properties of novel heat resistant polyimide nanocomposites derived from 14 H-dibenzo [a,j] xanthenes. J Polym Res 21:513. doi:10.1007/s10965-014-0513-5
Tuzen M, Soylak M (2009) Column solid-phase extraction of nickel and silver in environmental samples prior to their flame atomic absorption spectrometric determinations. J Hazard Mater 164:1428. doi:10.1016/j.jhazmat.2008.09.050
Yamini Y, Faraji M, Shariati S, Hassani R, Ghambarian M (2008) On-line metals preconcentration and simultaneous determination using cloud point extraction and inductively coupled plasma optical emission spectrometry in water samples. Anal Chim Acta 612:144. doi:10.1016/j.aca.2008.02.034
Tuzen M, Soylak M, Elci L (2005) Multi-element pre-concentration of heavy metal ions by solid phase extraction on Chromosorb108. Anal Chim Acta 548:101
Ngeontae W, Aeungmaitrepirom W, Tuntulani T (2007) Chemically modified silica gel with aminothioamidoanthraquinone for solid phase extraction and preconcentration of Pb(II), Cu(II), Ni(II), Co(II) and Cd(II). Talanta 71:1075. doi:10.1016/j.talanta.2006.05.094
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOCX 90.9 kb)
Rights and permissions
About this article
Cite this article
Bagtash, M., Yamini, Y., Tahmasebi, E. et al. Magnetite nanoparticles coated with tannic acid as a viable sorbent for solid-phase extraction of Cd2+, Co2+ and Cr3+ . Microchim Acta 183, 449–456 (2016). https://doi.org/10.1007/s00604-015-1667-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-015-1667-5