Abstract
We report on a simple method for the determination of traces of aluminum(III) in water at pH 7.4 by using silver nanoparticles (Ag-NPs) functionalized with 8-hydroxyquinoline-5-sulfonate. The modified Ag-NPs undergo (a) a distinct color change from yellow to deep orange, and (b) a strong fluorescence enhancement upon addition of Al(III). Both the ratio of absorbances at 530 and 392 nm, and the intensity of fluorescence at 492 nm can serve as the analytical information. The absorption-based calibration plot increases linearly in the 0.1 to 4.0 μM Al(III) concentration range. The detection limit is 2.0 nM which is much lower than the permissible level (7.4 μM) for drinking water as defined by the World Health Organization. The method was successfully applied to the determination of Al(III) in samples of lake water, tap water and boiler water, and the recoveries were from 98 to 105 %. The assay also was applied to the determination of Al(III) in living mouse myeloma cells via fluorescence imaging. A linear relationship was obtained between relative fluorescence intensity (F/F0) and the concentration of Al(III) in the 0.05 μM to 4 μM concentration range. The detection limit is 15 nM.

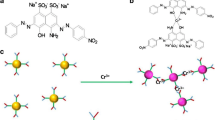
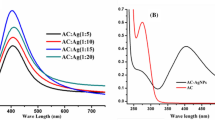
A colorimetric assay for the traces Al3+ using silver nanoparticles (Ag-NPs) functionalized with 8-hydroxyquinoline-5-sulfonic acid was introduced. The color change was ascribed to aggregation of Ag-NPs induced by Al3+.







Similar content being viewed by others
References
Klein GL (2005) Aluminum: new recognition of an old problem. Curr Opin Pharmacol 5:637–640
Kazi TG, Khan S, Baig JA, Kolachi NF, Afridi HI, Shah AQ (2010) Determination of trace quantity of aluminium in dialysate concentrates using solid phase and cloud point extraction methods. Anal Methods 2:558–563
House E, Esiri M, Forster G, Ince PG, Exley C (2012) Aluminium, iron and copper in human brain tissues donated to the medical research council's cognitive function and ageing study. Metallomics 4:56–65
Bohrer D, Heitmann U, Huang MD, Ross HB, Florek S, Welz B, Bertagnolli D (2007) Determination of aluminum in highly concentrated iron samples: Study of interferences using high-resolution continuum source atomic absorption spectrometry. Spectrochim Acta B 62:1012–1018
Rezaee M, Yamini Y, Khanchi A, Faraji M, Saleh A (2010) A simple and rapid new dispersive liquid–liquid microextraction based on solidification of floating organic drop combined with inductively coupled plasma-optical emission spectrometry for preconcentration and determination of aluminium in water samples. J Hazard Mater 178:766–770
Min PH, Nim OB, Hoon KJ, Wu Q, Hong HI, Deog JK, Cheal K, Jin HK (2011) Fluorescent chemosensor based-on naphthol–quinoline for selective detection of aluminum ions. Tetrahedron Lett 52:5581–5584
Xu Q, Du S, Jin GD, Li HB, Hu XY (2011) Determination of acetamiprid by a colorimetric method based on the aggregation of gold nanoparticles. Microchim Acta 173:323–329
Zhang YF, Li BX, Chen XL (2010) Simple and sensitive detection of dopamine in the presence of high concentration of ascorbic acid using gold nanoparticles as colorimetric probes. Microchim Acta 168:107–113
Jiang Z, Zhou L, Liang A (2011) Resonance scattering detection of trace melamine using aptamer-modified nanosilver probe as catalyst without separation of its aggregations. Chem Commun 47:3162–3164
Ravindran A, Mani V, Chandrasekaran N, Mukherjee A (2011) Selective colorimetric sensing of cysteine in aqueous solutions using silver nanoparticles in the presence of Cr3+. Talanta 85:533–540
Tashkhourian J, Hormozi MR, Khodaveisi J, Dashti R (2011) A novel photometric glucose biosensor based on decolorizing of silver nanoparticles. Sens Actuators B 158:185–189
Qi L, Shang Y, Wu FY (2012) Colorimetric detection of lead (II) based on silver nanoparticles capped with iminodiacetic acid. Microchim Acta 178:221–227
Liu J, Lu Y (2007) Colorimetric Cu2+ detection with a ligation DNAzyme and nanoparticles. Chem Commun 27:4872–4874
Yao Y, Tian D, Li H (2010) Cooperative binding of bifunctionalized and click-synthesized silver nanoparticles for colorimetric Co2+ sensing. ACS Appl Mater Inter 2:684–690
Ma YR, Niu HY, Zhang XL, Cai YQ (2011) Colorimetric detection of copper ions in tap water during the synthesis of silver/dopamine nanoparticles. Chem Commun 47:12643–12645
Zhan J, Wen L, Miao F, Tian D, Zhu X, Li H (2012) Synthesis of a pyridyl-appended calix[4]arene and its application to the modification of silver nanoparticles as an Fe3+ colorimetric sensor. New J Chem 36:656–661
Deng L, Zhou Z, Li J, Li T, Dong S (2011) Fluorescent silver nanoclusters in hybridized DNA duplexes for the turn-on detection of Hg2+ ions. Chem Commun 47:11065–11067
Jiang C, Guan Z, Lim SYR, Polavarapu L, Xu QH (2011) Two-photon ratiometric sensing of Hg2+ by using cysteine functionalized Ag nanoparticles. Nanoscale 3:3316–3320
Wang GL, Zhu XY, Jiao HJ, Dong YM, Li ZJ (2012) Ultrasensitive and dual functional colorimetric sensors for mercury (II) ions and hydrogen peroxide based on catalytic reduction property of silver nanoparticles. Biosens Bioelectron 31:337–342
Chen S, Fang YM, Xiao Q, Li J, Li SB, Chen HJ, Sun JJ, Yang HH (2012) Rapid visual detection of aluminium ion using citrate capped gold nanoparticles. Analyst 137:2021–2023
Li X, Wang J, Sun L, Wang Z (2010) Gold nanoparticle-based colorimetric assay for selective detection of aluminium cation on living cellular surfaces. Chem Commun 46:988–990
Muegge BD, Brooks S, Richter MM (2003) Electrochemiluminescence of tris(8- hydroxyquinoline- 5-sulfonic acid)aluminium(III) in aqueous solution. Anal Chem 75:1102–1105
Soroka K, Vithanage RS, Phillips DA, Walker B, Dasgupta PK (1987) Fluorescence properties of metal complexes of 8-hydroxyquinoline-5-sulfonic acid and chromatographic applications. Anal Chem 59:629–636
Swaile DF, Sepaniak MJ (1991) Determination of metal ions by capillary zone electrophoresis with on-column chelation using 8-hydroxyquinoline-5-sulfonic acid. Anal Chem 63:179–184
Zhu RH, Kok WT (1998) Determination of trace metal ions by capillary electrophoresis with fluorescence detection based on post-column complexation with 8-hydroxyquinoline-5-sulphonic acid. Anal Chim Acta 371:269–277
Zhang M, Ye BC (2011) Colorimetric chiral recognition of enantiomers using the nucleotide-capped silver nanoparticles. Anal Chem 83:1504–1509
(2004) Guidelines for drinking water quality.World Health Organization Geneva 3rd edn 301
Jiang XH, Wang BD, Yang ZY, Liu YC, Li TR, Liu ZC (2011) 8- Hydroxyquinoline- 5- carbaldehyde Schiff-base as a highly selective and sensitive Al3+ sensor in weak acid aqueous medium. Inorg Chem Commun 14:1224–1227
Abbaspour A, Esmaeilbeig AR, Jarrahpour AA AA, Khajeh B, Kia BR (2002) Aluminium(III)-selective electrode based on a newly synthesized tetradentate Schiff base. Talanta 58:397–403
Lu JS, Tian JY, Guo N, Wang Y, Pan YC (2011) Microemulsion extraction separation and determination of aluminium species by spectrofluorimetry. J Hazard Mater 185:1107–1114
Buratti M, Valla C, Pellegrino O, Rubino FM, Colombi A (2006) Aluminum determination in biological fluids and dialysis concentrates via chelation with 8-hydroxyquinoline and solvent extraction/fluorimetry. Anal Biochem 353:63–68
Tabrizi AB (2007) Cloud point extraction and spectrofluorimetric determination of aluminium and zinc in foodstuffs and water samples. Food Chem 100:1698–1703
Tontrong S, Khonyoung S, Jakmunee J (2012) Flow injection spectrophotometry using natural reagent from Morinda citrifolia root for determination of aluminium in tea. Food Chem 132:624–629
Acknowledgments
This work was financially supported by Jiangxi Province Natural Science Foundation (JXNSF No. 20132BAB203011).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 1.44 mb)
Rights and permissions
About this article
Cite this article
Shang, Y., Gao, D., Wu, F. et al. Silver nanoparticles capped with 8-hydroxyquinoline-5-sulfonate for the determination of trace aluminum in water samples and for intracellular fluorescence imaging. Microchim Acta 180, 1317–1324 (2013). https://doi.org/10.1007/s00604-013-1055-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-013-1055-y