Abstract
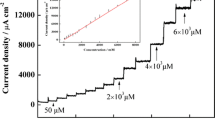
We report on a modified glassy carbon electrode (GCE) for sensing hydrogen peroxide (H2O2). It was constructed by consecutive electrochemical deposition of poly(anthranilic acid) and poly(diphenylamine sulfonate) on the GCE, followed by the deposition of copper oxide (CuO). The morphology and electrochemistry of the modified electrode was characterized by atomic force microscopy, X-ray diffraction, cyclic voltammetry, and electrochemical impedance spectroscopy. The catalytic performance of the sensor was studied with the use of differential pulse voltammetry under optimized conditions. This sensor displayed significantly better electrocatalytic activity for the reduction of H2O2 in comparison to a GCE without or with modification with CuO or polymer films alone. The response to H2O2 is linear in the range between 0.005 to ~11 mM, and the detection limit is 0.18 μM (at an S/N of 3).

A new bio-mimetic sensor, CuO/PANA@PSDS/GCE, was prepared, it exhibited a better electrocatalytic activity toward the reduction of the H2O2 compared with that of the CuO/GCE, PANA@PSDS/GCE, and GCE. Its increased catalytic response was due to the polyaniline doped (PANA@PSDS) film, which enlarges the specific surface area of the electrode, and increases the loading of the CuO nano-particles.







Similar content being viewed by others
References
Collins PG, Zettl A, Bando H, Thess A, Smalley RE (1997) Nanotube nanodevice. Science 278:100–102
Tian JQ, Li HL, Lu WB, Luo YL, Wang L, Sun XP (2011) Preparation of Ag nanoparticle-decorated poly(m-phenylenediamine) microparticles and their application for hydrogen peroxide detection. Analyst 136:1806–1809
Bockrath M, Cobden DH, McEuen PL, Chopra NG, Thess A (1997) Single-electron transport in ropes of carbon nanotubes. Science 275:1922–1925
Balamurugan A, Ho KC, Chen SM, Huang TY (2010) Electrochemical sensing of NADH based on meldola blue immobilized silver nanoparticles-conducting polymer electrode. Colloids Surf 362:1–7
Ding SN, Gao BH, Shan D, Sun YM, Cosnier S (2013) TiO2 nanocrystals electrochemiluminescence quenching by biological enlarged nanogold particles and its application for biosensing. Biosens Bioelectron 39:342–345
Muller R, Dutz S, Neeb A, Cato ACB, Zeisberger M (2013) Magnetic heating effect of nanoparticles with different sizes and size distributions. J Magn Magn Mater 328:80–85
Akman O, Kavas H, Baykal A, Toprak MS, Ali C, Aktas B (2013) Magnetic metal nanoparticles coated polyacrylonitrile textiles as microwave absorber. J Magn Magn Mater 327:151–158
Zhao WJ, Wang ZH, Li QM (2012) Fabrication of a nichrome electrode coated with silver microcrystals, and its application to sensing hydrogen peroxide. Anal Methods 4:1105–1109
Chen SH, Yuan R, Chai YQ, Hu FX (2013) Electrochemical sensing of hydrogen peroxide using metal nanoparticles: a review. Microchim Acta 180:15–32
Annamalai SK, Palani B, Pillai KC (2012) Highly stable and redox active nano copper species stabilized functionalized-multiwalled carbon nanotube/chitosan modified electrode for efficient hydrogen peroxide detection. Colloids Surf A 395:207–216
Zeng AP, Jin CY, Cho SJ, Seo HO, Kim YD, Lim DC, Kim DH, Hong BY, Boo JH (2012) Nickel nano-particle modified nitrogen-doped amorphous hydrogenated diamond-like carbon film for glucose sensing. Mater Res Bull 47:2713–2716
Cao F, Guo S, Ma HY, Yang GC, Yang SX, Gong J (2011) Highly sensitive nonenzymatic glucose sensor based on electrospun copper oxide-doped nickel oxide composite microfibers. Talanta 86:214–220
Zhu L, Shao G, Luo JK (2012) Numerical study of metal oxide Schottky type solar cells. Solid State Sci 14:857–863
Verma MK, Gupta V (2012) A highly sensitive SnO-CuO multilayered sensor structure for detection of H2S gas. Sens Actuators B 166–167:378–385
Hübner M, Simion CE, Tomescu-Stănoiu A, Pokhrel S, Bârsan N, Weimar U (2011) Influence of humidity on CO sensing with p-type CuO thick film gas sensors. Sens Actuators B 153:347–353
Yang J, Jiang LC, Zhang WD, Gunasekaran S (2010) A highly sensitive non-enzymatic glucose sensor based on a simple two-step electrodeposition of cupric oxide (CuO) nanoparticles onto multi-walled carbon nanotube arrays. Talanta 82:25–33
Priya S, Berchmans S (2012) Copper oxide-modified glassy carbon electrode prepared through copper hexacyanoferrate-G5-PAMAM dendrimer templates as electrocatalyst for carbohydrate and alcohol oxidation. J Solid State Electrochem 16:1527–1535
Park JH, Cho JH, Cho KH, Lee TW, Han HS, Shin CH (2012) CO oxidation over CuO catalysts prepared with different precipitants. Korean J Chem Eng 29:1151–1157
Wang BJ, Luo LQ, Ding YP, Zhao DS, Zhang QL (2012) Synthesis of hollow copper oxide by electrospinning and its application as a nonenzymatic hydrogen peroxide sensor. Colloids Surf B 97:51–56
Chen B, Wang H, Zhang HY, He ZX, Zhang SJ, Liu T, Zhou YZ (2012) A novel hydrogen peroxide sensor based on hemoglobin immobilized Pan-SiO2/DTAB composite film. J Mol Liq 171:23–28
Zhang Y, Luo HQ, Li NB (2011) Hydrogen peroxide sensor based on Prussian blue electrodeposited on (3-mercaptopropyl)-trimethoxysilane polymer-modified gold electrode. Bioprocess Biosyst Eng 34:215–221
Wang ZD, Sun SB, Hao XG, Ma XL, Guan GQ, Zhang ZG, Liu SB (2012) A facile electrosynthesis method for the controllable preparation of electroactive nickel hexacyanoferrate/polyaniline hybrid films for H2O2 detection. Sens Actuators B 171–172:1073–1080
Zhong HA, Yuan R, Chai YQ, Zhang Y, Wang CY, Jia F (2012) Non-enzymatic hydrogen peroxide amperometric sensor based on a glassy carbon electrode modified with an MWCNT/polyaniline composite film and platinum nanoparticles. Microchim Acta 176:389–395
Sheng QL, Wang MZ, Zheng JB (2011) A novel hydrogen peroxide biosensor based on enzymatically induced deposition of polyaniline on the functionalized grapheme-carbon nanotube hybrid materials. Sens Actuators B: Chem 160:1070–1077
Yang JP, Burkinshaw SM, Zhou J, Monkman AP, Brown PJ (2003) Fabrication and characteristics of 2-acrylamido-2-methyl-1-propanesulfonic acid-doped polyaniline hollow fibers. Adv Mater 15:1081–1084
Wang XL, Yang T, Feng YY (2009) A novel hydrogen peroxide biosensor based on the synergistic effect of gold-platinum alloy nanoparticles/polyaniline nanotube/chitosan nanocomposite membrane. Electroanalysis 21:819–825
Hao LP, Niu HJ, Zhao P, Zhang L, Sun Y, Wang C, Bai XD, Wang W (2012) Applications of poly(3,5-diaminobenzotrifluoride) as electrode in dye-sensitized solar cells and electrochromic devices. Electrochim Acta 68:103–109
Karyakin AA, Strakhova AK, Yatsimirsky AK (1994) Self-doped polyanilines electrochemically active in neutral and basic aqueous solutions.: Electropolymerization of substituted anilines. J Electroanal Chem 371:259–265
Yamamoto K, Taneichi D (1999) Electrochemical catalytic reduction of oxygen by a self-doped polyaniline-co-porphyrin complex-modified glassy carbon electrode. J Inorg Organomet Polym 9:231–243
Bartlett PN, Simon E (2000) Poly(aniline)-poly(acrylate) composite films as modified electrodes for the oxidation of NADH. Phys Chem Chem Phys 2:2599–2606
Shieh YT, Jung JJ, Lin RH, Yang CH, Wang TL (2012) Electrocatalytic activity of self-doped polyaniline. Electrochim Acta 70:331–337
Erdogan IY, Gullu O (2010) Optical and structural properties of CuO nanofilm: its diode application. J Alloys Compd 492:378–383
Ahmad R, Mehrnaz G (2012) Preparation and properties of semiconductor CuO nanoparticles via a simple precipitation method at different reaction temperatures. Opt Quantum Electron 44:313–322
Lin XY, Ni YN, Kokot S (2012) Voltammetric analysis with the use of a novel electro-polymerised graphene-nafion film modified glassy carbon electrode: simultaneous analysis of noxious nitroaniline isomers. J Hazard Mater 243:232–241
Huang KJ, Wang L, Li J, Liu YM, Electrochemical sensing based on layered MoS2-graphene composites. Sens Actuators B: Chem 178:671–677
Qin XY, Luo YL, Lu WB, Chang GH, Asiri AM, Al-Youbi AO, Sun XP (2012) One-step synthesis of Ag nanoparticles-decorated reduced graphene oxide and their application for H2O2 detection. Electrochim Acta 79:46–51
Acknowledgments
The authors gratefully acknowledge the financial support of this study by the National Natural Science Foundation of China (NSFC-21065007), the Education Department Science Foundation of Jiangxi Province (GJJ10037), and the State Key Laboratory of Food Science and Technology of Nanchang University (SKLF-ZZA-201302 and SKLF-ZZB-201303).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Song, H., Ni, Y. & Kokot, S. A glassy carbon electrode modified with poly(anthranilic acid), poly(diphenylamine sulfonate) and CuO nano-particles for the sensitive determination of hydrogen peroxide. Microchim Acta 180, 1263–1270 (2013). https://doi.org/10.1007/s00604-013-1053-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-013-1053-0