Abstract
The 20S proteasome is a multicatalytic enzyme complex responsible for intracellular protein degradation in mammalian cells. Its antigen level or enzymatic activity in blood plasma are potentially useful markers for various malignant and nonmalignant diseases. We have developed a method for highly selective determination of the 20S proteasome using a Surface Plasmon Resonance Imaging (SPRI) technique. It is based on the highly selective interaction between the proteasome’s catalytic β5 subunit and immobilized inhibitors (the synthetic peptide PSI and epoxomicin). Inhibitor concentration and pH were optimized. Analytical responses, linear ranges, accuracy, precision and interferences were investigated. Biosensors based on either PSI and epoxomicin were found to be suitable for quantitative determination of the proteasome, with a precision of ±10% for each, and recoveries of 102% and 113%, respectively, and with little interference by albumin, trypsin, chymotrypsin, cathepsin B and papain. The proteasome also was determined in plasma of healthy subjects and of patients suffering from acute leukemia. Both biosensors gave comparable results (2860 ng·mL-1 on average for control, and 42300 ng·mL-1 on average for leukemia patients).

The synthetic peptide aldehyde Z-Ile-Glu(OBut)-Ala-Leu-H (PSI) and a microbial α’,β’ epoxyketone peptide epoxomicin was used to develop SPRI biosensor for the highly selective determination of the 20S proteasome concentration, and to evaluate the sensor applicability for the determination of 20S proteasome in human blood plasma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Proteasomes are responsible for the degradation of most intracellular proteins in the cytoplasm and nucleus of all eukaryotic cells [1, 2]. Such proteins include mutated or damaged proteins, viral proteins, and many short-lived proteins that control cell cycle progression, apoptosis and the nuclear factor (NF)-κB transcriptional pathway; most of them are first polyubiquitinated by and then directed to the 26S proteasomes for degradation [2]. The 26S proteasome is a large multi-subunit complex (m. w. 2000 kDa) composed of a central 20S catalytic core particle (20S proteasome) and two 19S regulatory caps that recognize ubiquitinated proteins. The 20S proteasome (m. w. 700 kDa) has a cylindrical structure composed of two outer α-rings and two inner β-rings, each containing seven distinct subunits (α1−7β1−7β1−7α1−7) [3, 4] (Fig. 1a). The outer α-subunits control the entry of the substrate proteins into the central catalytic chamber and bind the regulators, while the three inner β subunits: β1, β2, and β5 contain an N-terminal catalytically active threonine (Thr) residue and harbor the caspase-like (Cas-L), trypsin-like (T-L) and chymotrypsin-like (ChT-L) activities, respectively [5, 6].
Of these, the ChT-L activity is the rate-limiting step in the degradation of intracellular proteins, including those that determine tumor growth and survival and mediate inflammatory conditions. A number of small molecule inhibitors that primarily target the ChT-L activity of the proteasome are described in the literature as possible anti-cancer and anti-inflammatory agents [reviewed in 7–10]. One of them, bortezomib, is the first proteasome inhibitor that was approved for the treatment of multiple myeloma patients [11], and is currently evaluated in clinical trials in patients with other hematological malignancies or advanced solid tumors [11–14]. Moreover, the 20S proteasomes released into the circulation (circulating proteasomes) are thought to be a novel biomarker for the prognosis and monitoring of patients suffering from various types of cancers, as well as other pathologies [reviewed in 15].
The most common method of detecting circulating proteasomes in plasma or serum is the enzyme-linked immunoabsorbent (ELISA) test employing antibodies directed against the constitutive α6 (C2) subunits of the 20S proteasome [16–23]. This method allows the quantitative determination of the intact 20S proteasome complexes, small proteasomal subunit fragments and free subunits in plasma or serum (i.e. total concentrations of circulating proteasomes). Unfortunately, techniques and antibodies used in this assay differ among investigators, so that results derived from different studies cannot be easily compared. An alternative method for circulating proteasome assay consists in measuring the ChT-L activity using the fluorogenic peptide substrate in the presence of an artificial activator sodium dodecyl sulfate (SDS) [24–27]. Although, the specificity of this assay was confirmed with highly selective proteasome inhibitors, the method allows for the detection of only the intact 20S proteasome complexes, since free catalytic subunits are enzymatically inactive.
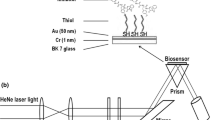
Surface Plasmon Resonance Imaging (SPRI) is a powerful technique for detecting biologically active species [28–32], including papain and lysosomal proteases [33–37]. So far, this method has not been applied to the determination of 20S proteasomes.
The aim of this study was to develop an SPRI biosensor for 20S proteasome, and to evaluate the sensor applicability for the determination of 20S proteasome concentration in human blood plasma. Additionally, Atomic Force Microscopy (AMF) was used for observation of a chip surface after creation of subsequent layers. AFM enables the observation of the surface of the biosensor at nm range. For the sensor development, a highly selective interaction between catalytic β5 subunit harboring the ChT-L activity and immobilized inhibitors: Z-Ile-Glu(OBut)-Ala-Leu-H (PSI) or epoxomicin was applied (Fig. 1b). Being a peptide aldehyde, PSI binds covalently to the N- terminal Thr1Oγ located on β5 subunits, and powerfully and reversibly blocks the ChT-L activity (IC50 250 nM) of the 20S proteasome [7, 9, 38]. Epoxomicin, an active microbial α’,β’ epoxyketone peptide, is a strong irreversible inhibitor of the 20S proteasome ChT-L activity (IC50 >200 nM) that binds covalently to the N-terminal catalytic Thr1Oγ to form a stable morpholino adduct [7–9, 39]. Both, PSI and epoxomicin have been commonly used to explore the role of the proteasomes in physiological and pathological processes [reviewed in 7, 9, 10].
Experimental
Reagents
20S proteasome (mammalian) (12 mg·mL-1 concentration in solution of composition: 20 mM Tris-HCl. pH 7.5, 1 mM EDTA, 1 mM DTT, 1 mM sodium azide) ( AFFINITI Research Products Ltd, Mamhead, United Kingdom, www.affinity-res.com), Z-Ile-Glu(OBut)-Ala-Leu-H (PSI) (C32H50O8N4, 618.77 Da), epoxomicin (EPO) (C28H50N4O7, 554.73 Da) (BIOMOL, Lörrach, Germany, www.biomol.com), cysteamine hydrochloride, cathepsin B from human placenta, trypsin from the human pancreas, α-chymotrypsin from the human pancreas, human albumin, papain from carica papaya, N-Ethyl-N’-(3-dimethyl aminopropyl) carbodiimide (EDC), HEPES sodium salt (all SIGMA, Steinheim, Germany, www.sigmaaldrich.com), N-Hydroxysuccinimide (NHS) (ALDRICH, Munich, Germany, www.sigmaaldrich.com) were used, as well as dichloroethane of HPLC grade (FLUKA, Munich, Germany, www.sigmaaldrich.com), absolute ethanol, sodium hydroxide, sodium chloride, sodium carbonate, sodium phosphate, potassium phosphate, sodium acetate, potassium chloride, magnesium chloride, (all POCh, Gliwice, Poland, www.poch.com.pl), acetic buffer pH = 4.99–5.57, HBS-ES solution pH = 7.4 (0.01 M HEPES, 0.15 M sodium chloride, 0.005% Tween 20, 3 mM EDTA), Phosphate Buffered Saline (PBS) pH = 7.4 (BIOMED, Lublin, Poland, www.biomed.lublin.pl), phosphate buffer pH = 7.17–8.04, and carbonate buffer pH = 8.50–9.86. Photopolimer ELPEMER SD 2054, hydrophobic protective paint SD 2368 UV SG-DG (PETERS, Kempen, Germany, www.peters.de) Chemicals were used as received. Aqueous solutions were prepared with filtered milliQ water (Simplicity®MILLIPORE).
Chip preparation
Gold chips were manufactured as described in a previous paper [33, 34]. Briefly, the gold surface of the chip was covered with photopolymer and hydrophobic paint. 9 × 12 free gold surfaces were obtained. Using this chip, nine different examined solutions can be simultaneously measured without mixing the tested solutions. Twelve single SPRI measurements can be performed from one solution.
Inhibitor immobilization
Chips were rinsed with ethanol and water and dried under a stream of nitrogen. They were then immersed in 20 mM of cysteamine ethanolic solutions for 2 h and after rinsing with ethanol and water dried again under a stream of nitrogen [34]. Epoxomicin or PSI at a concentration of 80 nM, each, were activated with NHS (50 mM) and EDC (200 mM) in a carbonate buffer (pH = 8.5) environment, and was then placed on the thiol-modified surface and incubated at 37 °C for 1 h.
SPRI measurements
SPRI measurements for the protein biosensor array were performed as described elsewhere [34]. The signal was measured twice on the basis of registered images, after immobilisation of the inhibitor and then after interaction with 20S proteasome. The SPRI signal, which is proportional to coupled biomolecules, was obtained from subtraction between the signal before and after interaction with a biomolecule for each spot separately. Thus, the SPRI signal was integrated over the spot area. NIH Image J version 1.32 software was used to evaluate the SPRI images in 2D form.
Measurement of 20S proteasome concentration in human blood plasma
Plasma samples from healthy adult donors (n = 9) and patients with acute leukemia (n = 9) were supplied by the Department of Hematology, Medical University of Bialystok, Poland. Patients and healthy donors who were included in the project were informed and their agreement was obtained. Approval for this study was obtained from the Local Ethics Committee. Plasma samples were 10-times diluted with PBS buffer.
The drops of plasma were transferred onto the chip surface for 10 min. A whole section of 12 spots was covered. The surface was washed with distilled water 10-times and after drying, the SPRI measurement was performed. An average value taken from 12 pairs of measurements was considered as a single result. Concentration was evaluated from the calibration curve of 20S proteasome.
Statistical analysis
All the results are given as the mean ± standard error of the mean (SEM). A value of p < 0.05 was considered significant.
Results and discussion
In order to select the best conditions for 20S proteasome determination by SPRI, the investigation was performed within an inhibitor concentration range of 20 to 200 nM at constant 20S proteasome concentration (8 nM). A series of 9 inhibitor solutions, pre-activated with NHS and EDC, were transferred onto different points of the chip surface, which was previously modified with cysteamine. Inhibitor (PSI or EPO) was immobilised during this treatment. The chip was then treated with the 20S proteasome solution (8 nM) for 10 min, rinsed with HBS-ES buffer and dried. The SPRI measurement was performed. Each solution was put on a field (each of 12 measuring points). The results are given in Fig. 2.
The obtained curve is an isotherm type, with the plateau of the signal for PSI or epoxomicin concentration above 60 nM. A concentration of inhibitor equal to 80 nM was selected as optimal for further investigation.
Influence of solution pH on the interaction process
The influence of pH of the 20S proteasome solution on the SPRI signal was studied for 9 different pH values (within the range 4.99–9.5), under conditions of constant concentrations of 20S proteasome (8 nM) and inhibitor (80 nM). Solutions of PSI or epoxomicin pre-activated with EDC and NHS, were transferred onto different points of a chip which was previously modified with cysteamine. In this manner the inhibitor was immobilised onto the chip surface. The chip was then treated with 20S proteasome solutions (8 nM) with different pH values, for 10 min. The results are shown in Fig. 3. As is apparent from the diagram, the maximum of the SPRI signal for inhibitor (PSI and EPO)-20S proteasome complex is located between 7.0 and 8.0. On this basis, the value of pH = 7.4 was selected as optimal for further investigation.
Analytical response of the sensor to 20S proteasome concentration. Calibration curves
The response of the analytical SPRI signal for 20S proteasome concentration was measured within a range of concentration between 2 and 25 nM. The chip surface was covered by a monolayer of cysteamine and a layer of immobilized PSI and epoxomicin (80 nM). The chip was then treated with the 20S proteasome solution. The time of interaction was 10 min. The experiments were performed at pH = 7.4. The obtained calibration curve is shown in Fig. 4. As can be seen in this figure, all calibration curves are of an isotherm type. The roughly linear sections of these curves are within a range of 2 and 10 nM and are useful analytically (Fig. 4). The plateau of the curve corresponds to saturation of active points of the sensor. The detection limit calculated on the 3 S.D. basis is equal to 0.16 nM for PSI inhibitor and 0.27 nM for epoxomicin.
Selectivity of the inhibitor-20S proteasome interaction
PSI is a highly selective inhibitor of the ChT-L activity of the proteasome. However, at 10-times higher concentration it can also inhibit cathepsin B and calpains. [7, 38]. By contrast, epoxomicin even at a concentration as high as 50 μM does not inhibit any proteases, including calpain, cathepsins, trypsin, chymotrypsin, papain [8, 39].
In order to be sure that the interaction between inhibitor (PSI or epoxomicin) and 20S proteasome is selective and that the sensor does not react with the other proteins, the chip containing immobilised inhibitor was treated with mixtures of 20S proteasome – albumin, 20S proteasome – trypsin, 20S proteasome – chymotrypsin, 20S proteasome – cathepsin B and 20S proteasome – papain mixtures. Various excesses of albumin within the range from 1:100 to 1:10000 were examined. Excesses of trypsin, chymotrypsin, cathepsin B and papain were 1:100 and 1:1000. Results are shown in Table 1. No influence of albumin, trypsin, chymotrypsin, cathepsin B or papain on the results of determination of 20S proteasome even at a 1000-fold excess using SPRI sensors was observed. Thus both sensors’ high selectivity was confirmed.
Precision of the developed method for 20 S proteasome determination
Precision of the developed method was tested under optimal conditions, i.e. pH = 7.4 and inhibitor (PSI or EPO) concentration at the chip preparation stage equal to 80 nM. The precision of 20S proteasome determination was tested for the concentration 4 nM. The results are shown in Table 2. Standard deviation and the confidence limit, assuming that the confidence level equals 95%, are relatively high. This indicates that the developed biosensors have a relatively low precision. However, the effect of relatively poor precision is compensated by a large number of repetitions, as the developed sensor is an array of 2 × 12 measuring spots. Therefore, recoveries of spikes are good (PSI) or acceptable (EPO).
Atomic force microscopy (AFM) measurements
AFM was used to confirm the creation of subsequent layers: gold, thiol (cysteamine), inhibitor (PSI or EPO) and 20S proteasome. Pictures were taken of bare gold (Fig. 5a), cysteamine (Fig. 5b), EPO (Fig. 5c) PSI (Fig 5d) and 20S proteasome on EPO and PSI (Fig. 5e and f). The presented AFM pictures confirm that the described stages of the creation of each layer on the biosensor surface has really taken place. This may be concluded on the basis of the creation of different structures after each stage.
Determination of 20S proteasome concentration in blood plasma samples
Blood plasma samples from 9 healthy volunteers and 9 patients suffering from acute leukaemia at the blast stage were analysed for 20S proteasome concentration using the developed sensors based on inhibitors PSI or EPO. The results of measurements were evaluated on the basis of a calibration graphs, and are given in Table 3. In order to have results comparable with the literature, the results were recalculated from nM to ng·mL-1. In healthy subjects, the mean value of plasma 20S proteasome concentration was established as 2870 ± 730 ng·mL-1 for the epoxomicin-based biosensor and 2850 ± 700 ng·mL-1 for the PSI biosensor. All patients with acute leukemias had increased plasma 20S proteasome concentration, with mean values being as high as 41600 ± 8570 ng·mL-1 for the epoxomicin-based biosensor and 43000 ± 10400 ng·mL-1 for the PSI biosensor. The finding that plasma 20S proteasome concentrations measured using two biosensors were very similar enhances the credibility of the results.
The results for healthy subjects are, in general, consistent with those obtained by other investigators, who, using ELISA-based assay have demonstrated that the average concentrations of plasma 20S proteasome in the controls are between 2100 and 2600 ng·mL-1 [17–19, 21]. However, it should be mentioned that other groups of investigators have demonstrated that 20S proteasome antigen concentration in plasma or serum from normal subjects was less than 400 ng·mL-1 [16, 20, 40]. Furthermore, the mean value of plasma 20S proteasome concentrations measured by SPRI in patients with acute leukemia was up to 15 times higher as compared to plasma of control persons, whereas the mean values of serum or plasma proteasome concentrations determined by ELISA in patients suffering from the same types of tumor was about 8-fold [16] or only 2-fold higher as compared to healthy subjects [18]. It is also worth noting that Wada et al [16] reported a large variability in 20S proteasome antigen concentration in the plasma of patients with acute leukemia (i.e. 2900 ± 2569 ng·mL-1). No such variability in elevated plasma 20S proteasome concentration was observed using SPRI biosensors. Based on this data, it appears that the SPRI technique can be more suitable and more clinically useful than ELISA-based assay for measurements of 20S proteasome concentration in human blood plasma.
Conclusions
Two selective SPRI array biosensors for the determination of 20S proteasome concentration have been developed. The sensors work on the basis of a highly selective reaction between immobilised PSI or epoxomicin inhibitors and 20S proteasome in solution. Under optimised conditions, both sensors exhibit selectivity to 20S proteasome, and the precision and accuracy is well suited to protein complex determination. The sensor can be used for the determination of 20S proteasome in human plasma, and possibly other biological material.
References
Coux O, Tanaka K, Goldberg AL (1996) Structure and functions of the 20S and 26S proteasomes. Annu Rev Biochem 65:801–847
Glickman MH, Ciechanover A (2002) The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol Rev 82:373–428
Baumeister W, Dahlmann B, Hergel R, Kopp F, Kuehn L, Pfeifer G (1988) Electron microscopy and image analysis of the multicatalytic proteinase. FEBS Lett 241:239–245
Groll M, Dietzel L, Lowe J, Stock D, Bochtler M, Bartnik H, Huber R (1997) Structure of 20S proteasome from yeast at 2,4 Å resolution. Nature 386:463–471
Groll M, Bochtler M, Brandstetter H, Clausen T, Huber R (2005) Molecular machines for protein degradation. Chem Bio Chem 6:222–256
Orlowski M, Wilk S (2000) Catalytic activities of the 20S proteasome, a multicatalytic proteinase complex. Arch Biochem Biophys 383:1–16
Kisselev AF, Goldberg AL (2001) Proteasome inhibitors: from research tools to drug candidates. Chem Biol 8:739–758
Groll M, Huber R (2004) Inhibitors of the eukaryotic 20S proteasome core particle: a structural approach. Biochim Biophys Acta 1695:33–44
de Bettignies G, Coux O (2010) Proteasome inhibitors: dozens of molecules and still counting. Biochimie 92(11):1530–1545
Myung J, Kim KB, Crews CM (2001) The ubiquitin-proteasome pathway and proteasome inhibitors. Med Res Rev 21(4):245–273
Adams J, Kauffman M (2004) Development of the proteasome inhibitor Velcade (Bortezomib). Canc Investig 22:304–311
Ludwig H, Khayat D, Giaccone G, Facon T (2005) Proteasome inhibition and its clinical prospects in the treatment of hematologic and solid malignancies. Cancer 104:1794–1807
Voorhees PM, Orlowski RZ (2006) The proteasome and proteasome inhibitors in cancer therapy. Annu Rev Pharmacol Toxicol 46:189–213
Crawford LJ, Walker B, Irvine AE (2008) Proteasome inhibitors: a therapeutic strategy for hematological malignancy. Front Biosci 13:4285–4296
Sixt SU, Dahlmann B (2008) Extracellular, circulating proteasomes and ubiquitin - incidence and relevance. Biochim Biophys Acta 1782(12):817–823
Wada M, Kosaka M, Saito S, Sano T, Tanaka K, Ichihara A (1993) Serum concentration and localization in tumor cells of proteasomes in patients with hematologic malignancy and their pathophysiologic significance. J Lab Clin Med 121:215–223
Lavabre-Bertrand T, Henry L, Carillo S, Guiraud I, Ouali A, Dutaud D, Aubry L, Rossi JF, Bureau JP (2001) Plasma proteasome level is a potential marker in patients with solid tumors and hemopoietic malignancies. Cancer 92:2493–2500
Dutaud D, Aubry L, Henry L, Levieux D, Hendil KB, Kuehn L, Bureau JP, Ouali A (2002) Development and evaluation of a sandwich ELISA for quantification of the 20S proteasome in human plasma. J Immunol Meth 260:183–193
Stoebner PE, Lavabre-Bertrand T, Henry L, Guiraud I, Carillo S, Dandurand M, Joujoux JM, Bureau JP, Meunier L (2005) High plasma proteasome levels are detected in patients with metastatic malignant melanoma. Br J Dermatol 152:948–953
Jakob C, Egerer K, Liebisch P, Türkmen S, Zavrski I, Kuckelkorn U, Heider U, Kaiser M, Fleissner C, Sterz J, Kleeberg L, Feist E, Burmester GR, Kloetzel PM, Sezer O (2007) Circulating proteasome levels are an independent prognostic factor for survival in multiple myeloma. Blood 109:2100–2103
Henry L, Lavabre-Bertrand T, Vercambre L, Ramos J, Carillo S, Guiraud I, Pouderoux P, Bismuth M, Valats JC, Demattei C, Duny Y, Chaze I, Funakoshi N, Bureau JP, Daurès JP, Blanc P (2009) Plasma proteasome level is a reliable early marker of malignant transformation of liver cirrhosis. Gut 58:833–838
Henry L, Lavabre-Bertrand T, Douche T, Uttenweiler-Joseph S, Fabbro-Peray P, Monsarrat B, Martinez J, Meunier L, Stoebner PE (2010) Diagnostic value and prognostic significance of plasmatic proteasome level in patients with melanoma. Exp Dermatol 19:1054–1059
Heubner M, Wimberger P, Dahlmann B, Kasimir-Bauer S, Kimmig R, Peters J, Wohlschlaeger J, Sixt SU (2011) The prognostic impact of circulating proteasome concentrations in patients with epithelial ovarian cancer. Gynecol Oncol 120:233–238
Ostrowska H, Hempel D, Holub M, Sokolowski J, Kloczko J (2008) Assessment of circulating proteasome chymotrypsin-like activity in plasma of patients with acute and chronic leukemias. Clin Biochem 41:1377–1383
Ma W, Kantarjian H, O’Brien S, Jilani I, Zhang X, Estrov Z, Ferrajoli A, Keating M, Giles F, Albitar M (2008) Enzymatic activity of circulating proteasomes correlates with clinical behavior in patients with chronic lymphocytic leukemia. Cancer 112:1306–1312
Ma W, Kantarjian H, Bekele B, Donahue AC, Zhang X, Zhang ZJ, O’Brien S, Estey E, Estrov Z, Cortes J, Keating M, Giles F, Albitar M (2009) Proteasome enzymatic activities in plasma as risk stratification of patients with acute myeloid leukemia and advanced-stage myelodysplastic syndrome. Clin Canc Res 15:3820–3826
Ma W, Kantarjian H, Zhang X, Wang X, Estrov Z, O’Brien S, Albitar M (2011) Ubiquitin-proteasome system profiling in acute leukemias and its clinical relevance. Leuk Res 35:526–533
Fernández-González A, Rychlowska J, Badía R, Salzer R (2007) SPR imaging as a tool for detecting mucin – anti-mucin interaction. Outline of the development of a sensor for near-patient testing for mucin. Microchim Acta 158:219–225
Chen H, Lee J, Jo WS, Jeong MH, Koh K (2011) Development of surface plasmon resonance immunosensor for the novel protein immunostimulating factor. Microchim Acta 172:171–176
Lee HJ, Yan Y, Marriott G, Corn RM (2005) Quantitative functional analysis of protein complexes on surfaces. J Physiol 563:61–71
Lee HJ, Nedelkov D, Corn RM (2006) Surface Plasmon Resonance Imaging measurements of antibody arrays for the multiplexed detection of low molecular weight protein biomarkers. Anal Chem 78:6504–6510
Fang S, Lee J, Wark AW, Corn RM (2006) Attomole microarray detection of microRNAs by nanoparticle-amplified SPR Imaging measurements of surface polyadenylation reactions. J Am Chem Soc 128:14044–14046
Gorodkiewicz E (2007) The Surface Plasmon Resonance Imaging sensor for papain based on immobilized cystatin. Protein Pept Lett 14:443–445
Gorodkiewicz E (2009) Surface Plasmon Resonance Imaging sensor for cathepsin determination based on immobilized cystatin. Protein Pept Lett 16:1379–1385
Gorodkiewicz E, Regulska E, Roszkowska-Jakimiec W (2010) Determination of the active form concentration of cathepsins D and B by SPRI biosensors. J Lab Diagn 46:107–109
Gorodkiewicz E, Regulska E (2010) SPR imaging biosensor for aspartyl cathepsins: sensor development and application for biological material. Protein Pept Let 17:1148–1154
Gorodkiewicz E, Regulska E, Wojtulewski K (2011) Development of an SPR imaging biosensor for determination of cathepsin G in saliva and white blood cells. Microchim Acta 173:407–413
Wilk S, Fiqueiredo-Pereira ME (1993) Synthetic inhibitors of the multicatalytic proteinase complex (proteasome). Enzyme Protein 47:306–313
Meng L, Mohan R, Kwok BHB, Elofsson M, Sin N, Crews CM (1999) Epoxomicin, a potent and selective proteasome inhibitor, exhibits in vivo anti-inflammatory activity. Proc Natl Acad Sci USA 96:10406–10408
Egerer K, Kuckelkorn U, Rudolph PE, Rückert JC, Dörner T, Burmester GR, Kloetzel PM, Feist E (2002) Circulating proteasomes are markers of cell damage and immunologic activity in autoimmune diseases. J Rheumatol 29(10):2045–2052
Acknowledgements
Doctor J.Svieklo is greatly thanked for offering invaluable help with the Atomic Force Microscope. Prof. J. Kloczko from the Department of Hematology of Medical University of Bialystok are greatly thanked for providing the blood plasma samples. Very fruitful consultations with Professor Zenon Lukaszewski from Poznan University of Technology are greatly appreciated.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Gorodkiewicz, E., Ostrowska, H. & Sankiewicz, A. SPR imaging biosensor for the 20S proteasome: sensor development and application to measurement of proteasomes in human blood plasma. Microchim Acta 175, 177–184 (2011). https://doi.org/10.1007/s00604-011-0656-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-011-0656-6