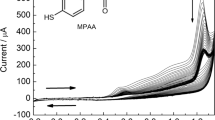
Abstract
A stepwise strategy is reported for the design of a meditor-free amperometric tyrosinase biosensor. It is based on the azide-alkyne click reaction and carbodiimide coupling. Firstly, azide-terminated alkane thiols monolayers were self-assembled on the Au electrode surface. Then, nitrophenyl groups were covalent attached to the self-assembled monolayers (SAMs) via the click reaction of copper(I)-catalyzed 1,3-dipolar cycloadditions of azide-alkyne. Finally, the nitrophenyl group terminated SAMs were converted to aminophenyl-terminated interface by electrochemical reduction, and tyrosinase was covalent immobilized onto the Au electrode via carbodiimide reaction. Based on the stepwise strategy, a meditor-free amperometric tyrosinase biosensor was farbricated, and it showed good electrocatalytic reduction ability toward phenol, pyrocatechol and m-Cresol. Their linear ranges were over the range of 0.2 to 15.0 μmol·L−1, 0.2 to 73.0 μmol·L−1, and 0.2 to 33.0 μmol·L−1, respectively.





Similar content being viewed by others
References
Castillo M, Domingues R, Alpendurada MF, Barcelo D (1997) Persistence of selected pesticides and their phenolic transformation products in natural waters using off-line liquid solid extraction followed by liquid chromatographic techniques. Anal Chim Acta 353:133
Gatti R, Gioia MG, Di Pietra AM, Cavrini V (2001) Analysis of phenols in pharmaceuticals by liquid chromatography after pre-column labelling and on-line post-column photochemical derivatization. Anal Chim Acta 447:89
Naczk M, Shahidi F (2004) Extraction and analysis of phenolics in food. J Chromatogr A 1054:95
Wang J, Zhou ND, Zhu ZQ, Huang JY, Li GX (2007) Detection of flavonoids and assay for their antioxidant activity based on enlargement of gold nanoparticles. Anal Bioanal Chem 388:1199
Liu XJ, Chen T, Hoshi T, Kashiwagi Y, Anzai J, Li GX (2005) Electrochemical determination of cinnamtannin b1 with a pyrolytic graphite electrode. Microchim Acta 150:73
Zhou H, Sun ZY, Hoshi T, Kashiwagi Y, Anzai J, Li GX (2005) Electrochemical studies of danthron and the DNA–danthron interaction. Biophys Chem 114:21
Takashima RW, Kaneto K (2004) Amperometric phenol biosensor based on covalent immobilization of tyrosinase onto an electrochemically prepared novel copolymer poly (N-3-aminopropyl pyrrole-co-pyrrole) film. Sens Actuators B 102:271
Li N, Xue MH, Yao H, Zhu JJ (2005) Reagentless biosensor for phenolic compounds based on tyrosinase entrapped within gelatine film. Anal Bioanal Chem 383:1127
Tsai YC, Chiu CC (2007) Amperometric biosensors based on multiwalled carbon nanotube-Nafion-tyrosinase nanobiocomposites for the determination of phenolic compounds. Sens Actuators B 125:10
Njagi J, Andreescu S (2007) Stable enzyme biosensors based on chemically synthesized Au–polypyrrole nanocomposites. Biosens Bioelectron 23:168
Wang B, Zhang J, Dong S (2000) Silica sol–gel composite film as an encapsulation matrix for the construction of an amperometric tyrosinase-based biosensor. Biosens Bioelectron 15:397
Liu ZJ, Liu BH, Kong JL, Deng JQ (2000) Probing trace phenols based on mediator-free alumina sol-gel derived tyrosinase biosensor. Anal Chem 72:4707
Kochana J, Gala A, Parczewski A, Adamski J (2008) Titania sol–gel-derived tyrosinase-based amperometric biosensor for determination of phenolic compounds in water samples. Examination of interference effects. Anal Bioanal Chem 391:1275
Rajesh KK (2005) A new tyrosinase biosensor based on covalent immobilization of enzyme on N-(3-aminopropyl) pyrrole polymer film. Curr Appl Phys 5:178
Zhou YL, Tian RH, Zhi JF (2007) Amperometric biosensor based on tyrosinase immobilized on a boron-doped diamond electrode. Biosens Bioelectron 22:822
Kolb HC, Finn MG, Sharpless KB (2001) Click chemistry: diverse chemical function from a few good reactions. Angew Chem Int Ed 40:2004
Wang Q, Chittaboina S, Barnhill HN (2005) Advances in 1, 3-dipolar cycloaddition reaction of azides and alkynes—a prototype of click chemistry. Lett Org Chem 2:293
Sun XL, Stabler CL, Cazalis CS, Chaik EL (2006) Carbohydrate and protein immobilization onto solid surfaces by sequential diels−alder and azide−alkyne cycloadditions. Bioconjug Chem 17:52
Li Y, Zhang WX, Chang J, Chen JC, Li GT, Ju Y (2008) “Click” on conducting polymer coated electrodes versatile platform for modification of electrode surface. Macromol Chem Phys 209:322
Collman JP, Devaraj NK, Chidsey CED (2004) “Clicking” functionality onto electrode surfaces. Langmuir 20:1051
Zhang Y, Luo SZ, Tang YJ, Yu L, Hou KY, Cheng JP, Zeng XQ, Wang PG (2006) Carbohydrate−protein interactions by “clicked” carbohydrate self-assembled monolayers. Anal Chem 78:2001
Brennan JL, Hatzakis NS, Tshikhudo TR, Dirvianskyte N, Razumas V, Patkar S, Vind J, Svendsen A, Nolte RJM, Rowan AE, Brust M (2006) Bionanoconjugation via click chemistry: the creation of functional hybrids of lipases and gold nanoparticles. Bioconjug Chem 17:1373
Wang LW, Tian Y, Ran Q, Hu ZC, Xu JJ, Xian YZ, Peng R, Jin LT (2009) Covalent grafting nitrophenyl group on Au surface via click reaction: assembling process and electrochemical behaviors. Electrochem Commun 11:339
Rostovtsev VV, Green JG, Fokin VV, Sharpless KB (2002) A stepwise Huisgen cycloaddition process: copper(i)-catalyzed regioselective ligation of azides and terminal alkynes. Angew Chem Int Ed 41:2596
Mendes PM, Christman KL, Parthasarathy P, Schopf E, Ouyang JY, Yang Y, Preece JA, Maynard HD, Chen Y, Stoddart JF (2007) Electrochemically controllable conjugation of proteins on surfaces. Bioconjug Chem 18:1919
Allongue P, Delamar M, Desbat B, Fagebaume O, Hitmi R, Pinson J, Savéant JM (1997) Covalent modification of carbon surfaces by aryl radicals generated from the electrochemical reduction of diazonium salts. J Am Chem Soc 119:201
Ortiz B, Saby C, Champagne GY, Belanger D (1998) Electrochemical modification of a carbon electrode using aromatic diazonium salts. 2. Electrochemistry of 4-nitrophenyl modified glassy carbon electrodes in aqueous media. J Electroanal Chem 455:75
Wang SF, Tan YM, Zhao DM, Liu GD (2008) Amperometric tyrosinase biosensor based on Fe3O4 nanoparticles–chitosan nanocomposite. Biosens Bioelectron 23:1781
Liu ZM, Liu J, Shen GL, Yu RQ (2006) A reagentless tyrosinase biosensor based on 1, 6-hexanedithiol and nano-Au self-assembled monolayers. Electroanalysis 18:1572
Sarver RW, Krueger WC (1991) An infrared and circular dichroism combined approach to the analysis of protein secondary structure. Anal Biochem 199:61
Kauppinen JK, Moffat DJ, Mantsch HH, Cameron DG (1981) Fourier self-deconvolution: a method for resolving intrinsically overlapped bands. Appl Spectrosc 35:271
Zhou H, Liu L, Yin K, Liu SL, Li GX (2006) Electrochemical investigation on the catalytic ability of tyrosinase with the effect of nano titanium dioxide. Electrochem Commun 8:1168
Kertesz D, Zito R (1965) Mushroom polyphenoloxidase purification and general properties. Biochim Biophys Acta 96:447
Seo SY, Sharma VK, Sharma N (2003) Mushroom tyrosinase: recent prospects. J Agric Food Chem 51:2837
Yu JH, Liu SQ, Ju HX (2003) Mediator-free phenol sensor based on titania sol–gel encapsulation matrix for immobilization of tyrosinase by a vapor deposition method. Biosens Bioelectron 19:509
Li YF, Liu ZM, Liu YL, Yang YH, Shen GL, Yu RQ (2006) A mediator-free phenol biosensor based on immobilizing tyrosinase to ZnO nanoparticles. Anal Biochem 349:33
Carralero V, Mena ML, Gonzalez-Cortés A, Yáñez-Sedeño P, Pingarrón JM (2006) Development of a high analytical performance-tyrosinase biosensor based on a composite graphite–Teflon electrode modified with gold nanoparticles. Biosens Bioelectron 22:730
Chen LY, Gu BX, Zhu GP, Wu YF, Liu SQ, Xu CX (2008) Electron transfer properties and electrocatalytic behavior of tyrosinase on ZnO nanorod. J Electroanal Chem 617:7
Sanz VC, Mena ML, González-Cortés A, Yáñez-Sedeño P, Pingarrón JM (2005) Development of a tyrosinase biosensor based on gold nanoparticles-modified glassy carbon electrodes application to the measurement of a bioelectrochemical polyphenols index in wines. Anal Chim Acta 528:1
Cheng YX, Liu YJ, Huang JJ, Li K, Xian YZ, Zhang W, Jin LT (2009) Amperometric tyrosinase biosensor based on Fe3O4 nanoparticles-coated carbon nanotubes nanocomposite for rapid detection of coliforms. Electrochim Acta 54:2588
Gu BX, Xu CX, Zhu GP, Liu SQ, Chen LY, Li XS (2009) Tyrosinase immobilization on Zno nanorods for phenol detection. J Phys Chem B 113:377
Yildiz HB, Castillo J, Guschin DA, Toppare L, Schuhmann W (2007) Phenol biosensor based on electrochemically controlled integration of tyrosinase in a redox polymer. Microchim Acta 159:27
Zhang YC (2010) Electro-induced covalent cross-linking of chitosan and formation of chitosan hydrogel films: its application as an enzyme immobilization matrix for use in a phenol sensor. Anal Chem 82:5275
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 20875031), Shanghai Rising-Star Program (09QH1400800), New Century Excellent Talents in University (NCET-09-0357) and Shanghai Municipal Education Commission (No. 08ZZ25).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, L., Ran, Q., Tian, Y. et al. Covalent grafting tyrosinase and its application in phenolic compounds detection. Microchim Acta 171, 217–223 (2010). https://doi.org/10.1007/s00604-010-0433-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-010-0433-y