Abstract
Purpose
To investigate the clinical indications and prognostic significance of surgical interventions after chemotherapy using trastuzumab-containing regimens for patients with human epidermal growth factor receptor 2 (HER2)-positive advanced gastric cancer (AGC).
Methods
A total of 146 patients with AGC who underwent chemotherapy were enrolled in this retrospective study. Tumors with an immunohistochemistry (IHC) score of 3 + or an IHC score of 2 + plus fluorescence in situ hybridization positivity were defined as HER2-positive AGC. We devised a scoring system for predicting prognosis associated with conversion surgery.
Results
Thirty-three patients received trastuzumab-based chemotherapy for HER2-positive tumors. Multivariate analyses identified advanced age, peritoneal dissemination, histologically undifferentiated tumors, and tumor response of progressive disease as independent prognostic factors for a worse prognosis. Twelve patients with HER2-positive AGC underwent conversion surgery. The conversion surgery group of patients with HER2-positive AGC had a better prognosis than the chemotherapy-alone group. A prognostic scoring system based on age, peritoneal dissemination, and histological type was significantly correlated with the presence or absence of conversion surgery and the prognosis of patients with HER2-positive AGC.
Conclusions
Our scoring system has the clinical potential to predict prognosis associated with conversion surgery after trastuzumab-containing chemotherapy for patients with HER2-positive AGC.
Similar content being viewed by others
Introduction
Gastric cancer is the fifth most common malignancy and the second-leading cause of cancer-related death worldwide [1, 2]. However, with the rapid development of chemotherapy, the prognosis of patients with unresectable advanced gastric cancer (AGC) has improved dramatically. In particular, the Trastuzumab for Gastric Cancer (ToGA) trial demonstrated the clinical efficacy of trastuzumab as a first-line regimen for patients with human epidermal growth factor receptor 2 (HER2)-positive unresectable advanced or recurrent gastric cancer [3]. Consequently, the clinical application of trastuzumab has greatly impacted the strategic management of patients on chemotherapy. The 2018 Japanese Gastric Cancer Treatment Guidelines are currently applied in clinics, and strongly recommend trastuzumab-based chemotherapy for patients with HER2-positive AGC [4].
In recent years, conversion surgery has attracted attention as a promising tool for improving the prognosis of responders with AGC after chemotherapy [5,6,7]. However, few studies have assessed the optimal timing of surgical interventions and the selection of candidates for conversion surgery. Therefore, the prognostic impact of conversion surgery and its clinical indication remain unclear in patients with HER-2-positive AGC who receive trastuzumab-based chemotherapy. A prognostic scoring system determined by pre-therapeutic factors that can be applied to selected candidates suitable for conversion surgery after chemotherapy would be valuable for the effective management of patients with HER2-positive AGC.
The purpose of the present study was to investigate tumor response, the presence or absence of conversion surgery, and the prognosis of patients with HER2-positive AGC. Furthermore, we propose a prognostic scoring system for selecting surgical candidates from responders after trastuzumab-based chemotherapy for HER2-positive AGC.
Methods
Patients
We reviewed a total of 146 patients with unresectable AGC, who underwent chemotherapy at Kagoshima University Hospital (Kagoshima, Japan) between September 2010 and December 2020. Patients with synchronous and metachronous cancer in other organs and those with disease recurrence were excluded from the present study. All patients underwent blood examinations, esophagogastroduodenoscopy, endoscopic ultrasonography, and computed tomography (CT) before receiving chemotherapy. Patients were categorized and staged based on the tumor–node–metastasis classification for gastric carcinoma established by the International Union Against Cancer [8].
Table 1 shows the clinicopathological features of the enrolled patients. The study included 94 men and 52 women with a mean age of 65.9 years (age range, 30–87 years). Among the 146 patients, 104, 28, 12, and 2 had metastases at 1, 2, 3, and 4 distant metastatic sites, respectively. Furthermore, 33 and 89 patients had liver metastasis and peritoneal dissemination, respectively. Differentiated and undifferentiated tumors were identified in 36 and 110 patients, respectively. This study was approved by the Ethics Committee of Kagoshima University (approval number: 200043).
Immunohistochemistry and fluorescence in situ hybridization for the assessment of HER2 expression
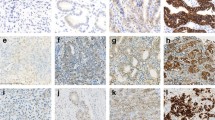
The tumor biopsy specimens obtained prior to chemotherapy were subjected to immunohistochemistry (IHC) analysis. All paraffin-embedded specimens were cut into 4-µm-thick slices and transferred to a slide. IHC was conducted using the Hercept test kit (Dako, Carpinteria, CA) based on the protocol recommended by the manufacturer. According to the Hercept test scoring criteria, staining intensity was scored as 0, 1 + , 2 + , or 3 + [3].
Fluorescence in situ hybridization (FISH) was conducted using the Abbott PathVysion HER2 DNA Probe Kit protocol (Abbott Laboratories, Abbott Park, Des Plaines, IL) according to the manufacturer’s instructions. HER2 gene amplification was assessed by calculating the number of HER2 and centromere enumerator probe 17 (CEP 17) signals in 20 adjacent interphase tumor cell nuclei, which were examined under a fluorescent microscope with the appropriate filters. A positive HER2 gene amplification status was defined as a HER2:CEP17 ratio of ≥ 2.0 [3].
In the present study, patients who had tumors with an IHC score of 3 + or an IHC score of 2 + plus FISH positivity were defined as having HER2-positive gastric cancer [3].
Assessment of tumor response to chemotherapy and histological response
Tumor response to chemotherapy was assessed once every three cycles and was determined based on the Response Evaluation Criteria in Solid Tumors (RECIST) [9]. This study grouped tumor response into the following two categories: progressive disease (PD) and non-PD.
Residual tumor status and the histological response at the primary tumor site were classified according to the Japanese classification of gastric carcinoma [10]. Accordingly, the surgical status for the assessment of residual tumors was categorized into R0 (no residual tumor), R1 (microscopic residual tumor), and R2 (macroscopic residual tumor). The histological tumor response was grouped into grades 0 (no effect), 1a (very slight effect), 1b (slight effect), 2 (considerable effect), and 3 (complete response).
Clinical indication for conversion surgery after chemotherapy
Conversion surgery after chemotherapy was clinically indicated for patients with a performance status (PS) of 0–2 and a tumor status predicted to achieve R0 curative resection. The decision about suitability for surgical treatment was also based on the patient’s health condition and the physician’s discretion.
Statistical analysis
The relationship between the score obtained using the prognostic scoring system and the presence or absence of conversion surgery was assessed using the chi-squared test. Survival time was defined as the duration from chemotherapy initiation to death or last follow-up. Kaplan–Meier survival curves were generated, and prognostic differences were assessed using the log-rank test. Prognostic factors were evaluated using univariate and multivariate analyses (Cox proportional hazards regression modeling). All data were analyzed using JMP14 (SAS Institute Inc., Cary, NC, USA). A p value of < 0.05 was considered significant.
Results
Characteristics of the patients with HER2-positive AGC
Among the 146 patients enrolled, the IHC scores denoting HER2 status of 46, 37, 30, 14, and 19 patients were as follows: 0, 1 + , 2 + plus FISH negativity, 2 + plus FISH positivity, and 3 + , respectively (Table 1). Accordingly, 33 patients (22.6%) had HER2-positive tumors.
Table 2 summarizes the clinicopathological factors of the 33 patients (age range 37–82 years; mean age 66.1 years) with HER2-positive AGC. Among the 33 patients, 3 and 30 had type 4 and type non-4 tumors, respectively. Moreover, 10 and 11 patients had liver metastasis and peritoneal dissemination, respectively. Staging laparoscopy before chemotherapy was done for 12 of 33 patients with HER2-positive AGC. Five patients had positive peritoneal cytology (CY1). The histological type of tumor was differentiated in 13 patients and undifferentiated in 20 patients. All patients received trastuzumab-based chemotherapy.
Tumor response to trastuzumab-based chemotherapy and prognosis
Among the 33 patients with HER2-positive AGC who received trastuzumab-based chemotherapy, 5 and 28 showed PD and non-PD, respectively, as per RECIST. Therefore, the disease control rate was 84.8% (28/33). Patients with HER2-positive AGC who showed PD and non-PD had a median survival time (MST) of 90 and 754 days, respectively (Fig. 1). The survival differences based on tumor responses were significant for patients with HER2-positive AGC (p < 0.0001).
Univariate and multivariate analyses of survival in patients with HER2-positive AGC
For univariate and multivariate analyses, the cutoff value of age was set at 70 years, based on the median age. Univariate analysis of patients with HER2-positive AGC identified that age (< 70 vs. ≥ 70 years), macroscopic type (type non-4 vs. type 4), peritoneal dissemination (P0 vs. P1), histological type (differentiated vs. undifferentiated), and tumor response (non-PD vs. PD) were significantly related to survival (p = 0.0207, p = 0.0116, p = 0.0117, p = 0.0020, and p = 0.0002, respectively; Table 3). Multivariate analysis revealed that age, peritoneal dissemination, histological type, and tumor response were independent prognostic factors (p = 0.0028, p = 0.0321, p = 0.0026, and p = 0.0033, respectively; Table 3).
Conversion surgery after chemotherapy and pathological findings in patients with HER2-positive AGC
Among the 33 patients with HER2-positive AGC, 12 (36.4%) underwent conversion surgery after chemotherapy. The median number of courses of trastuzumab-based chemotherapy before conversion surgery was 5 (3–17). Table 4 details the surgical procedures and pathological findings. Proximal gastrectomy, distal gastrectomy, total gastrectomy, and esophagectomy were performed in three (25.0%), four (33.3%), four (33.3%), and one (8.3%) patients, respectively. Furthermore, one (8.3%), four (33.3%), three (25.0%), and four (33.3%) patients underwent D1, D1 + , D2, and D2 + lymphadenectomy, respectively. Four (33.3%), three (25.0%), two (16.7%), and three (25.0%) patients had pathological T1, T2, T3, and T4 tumors, respectively. Moreover, seven (58.3%), two (16.7%), two (16.7%), and one (8.3%) patients had pathological N0, N1, N2, and N3 stage disease, respectively. R0 curative resection was achieved in all patients (n = 12). The histological tumor response analysis revealed that 10 and 2 patients had grade 1a and 1b disease, respectively. Among the 12 patients who underwent conversion surgery, 10 (83.3%) received adjuvant chemotherapy using S-1.
Table 5 shows the relationship between the presence or absence of conversion surgery and clinicopathological factors. Conversion surgery was significantly associated with age, the depth of tumor invasion, and histological type (p = 0.0374, p = 0.0152, and p = 0.0265, respectively). The 5-year overall survival (OS) rates of patients with HER2-positive AGC who underwent conversion surgery vs. those who received chemotherapy alone were 68.6% and 5.6%, respectively (p = 0.0002; Fig. 2). Furthermore, the 5-year OS rates after conversion surgery was 69.8% for the patients who underwent conversion surgery (Fig. 3).
Prognostic scoring system to predict suitability of clinical candidates for conversion surgery among patients with HER2-positive AGC
We established a prognostic scoring system to pre-therapeutically predict the suitability of candidates for conversion surgery after trastuzumab-based chemotherapy. This system consisted of three pre-therapeutic factors based on multivariate analysis of prognosis. Each factor was given a score of 1 point. The three factors were as follows: ≥ 70 years, the presence of peritoneal dissemination (P1), and undifferentiated tumor type. Accordingly, because the total score ranged from 0 to 3 points, patients were categorized into four groups.
This scoring system revealed 7 (21.2%), 9 (27.3%), 14 (42.4%), and 3 (9.1%) patients with prognostic scores of 0, 1, 2, and 3 points, respectively. The prognostic score correlated significantly with the presence or absence of conversion surgery (p = 0.0065; Table 6). All patients who scored 0 points were alive at the time of writing this article (Fig. 4). Patients with scores of 1, 2, and 3 points had MSTs of 757, 478, and 169 days, respectively. There were significant prognostic differences among patients with each score, except for patients with scores of 2 vs. 3 (p < 0.05; Fig. 4).
Discussion
Currently, trastuzumab-based chemotherapy is recommended as a first-line treatment for patients with HER2-positive AGC [4]. However, the incidence of HER2 gene amplification and/or protein overexpression in patients with AGC has been reported to range from 7 to 34% [11,12,13,14]. The low HER2 positivity rate among patients with AGC implies that trastuzumab-based chemotherapy may have limited benefit for AGC. Therefore, we must establish if conversion surgery after trastuzumab-based chemotherapy is a promising tool for improving the prognosis of patients with HER2-positive AGC. To our knowledge, this is the first study to develop and evaluate a prognostic scoring system for selecting suitable candidates for conversion surgery from among patients with HER2-positive AGC being treated with trastuzumab-based chemotherapy.
In the clinical management of patients with HER2-positive AGC, changes in HER2 status during trastuzumab-based chemotherapy have been highlighted in relation to drug resistance to subsequent anti-HER2 chemotherapy. Seo et al. [15] enrolled 48 patients with HER2-positive AGC treated with trastuzumab-containing first-line chemotherapy and investigated HER2 expression retrospectively, using IHC and/or FISH in tumor specimens at baseline and after PD to chemotherapy. They reported that tumor specimens from 14 patients (29.2%) who showed PD response (disease progression) to trastuzumab-based chemotherapy, exhibited HER2-loss status [15]. Moreover, patients with a stable HER2 status had a response rate of 44% and a median progression-free survival (PFS) of 2.7 months, whereas those with HER2-loss conversion were non-responsive and had a shorter PFS [15]. Similarly, Saeki et al. [16] assessed HER2 status retrospectively in re-biopsied specimens obtained from patients with HER2-postive AGC after a PD response to trastuzumab-containing first-line chemotherapy. They found that the incidence of HER2-loss status was 60.6% in that patient population (20/33). These results may suggest the limitation of trastuzumab-based chemotherapy alone in the clinical management of patients with HER2-positive AGC. Collectively, the above findings imply that therapeutic strategies including surgical interventions may improve the prognosis of patients with HER2-positive AGC.
Recent studies have demonstrated the prognostic utility of conversion surgery after chemotherapy in patients with stage IV AGC [5,6,7, 17,18,19]. Beom et al. [17] reviewed clinicopathological factors and prognosis retrospectively in 101 patients with stage IV AGC (unknown HER2 status), who were treated with systemic chemotherapy followed by gastrectomy, and reported that the median OS time was 26.0 months. Our study indicated that the median OS time was not reached in the conversion surgery group of patients with HER2-positive AGC. Surprisingly, in the present study, the 5-year OS rate in the conversion surgery group with HER2-positive AGC was 68.6%. Hayano et al. [18] examined prognostic data retrospectively in six patients with HER2-positive AGC, who underwent conversion surgery after trastuzumab-containing chemotherapy, and found that the 3-year survival rate was 66.7%. These findings imply that patients with HER2-positive AGC may be more suitable candidates for conversion surgery after trastuzumab-based chemotherapy, with greater improvements in prognosis, than those with HER2-negative AGC.
In this study, one patient underwent esophagectomy with lymphadenectomy (Table 4). The patient had a type 2 tumor of the esophagogastric junction and CT indicated swelling of the upper thoracic paraesophageal lymph node (station no. 105). Tubular adenocarcinoma with HER2 score of 3 + was identified by pathological examination of station no. 105 obtained by endoscopic ultrasound guided fine needle aspiration and a clinical diagnosis of Siewert type II esophagogastric junction adenocarcinoma of HER2-positive (T2N0M1) was made. The patient showed a complete response after three courses of trastuzumab-based chemotherapy and underwent conversion surgery. Pathological examination revealed that the primary tumor invaded the mucosa (ypT1a), indicating histological grade 1a. No tumor cells were seen in the dissected lymph nodes including station no. 105 (ypN0). Accordingly, a pathological diagnosis of stage IA (ypT1aN0M0) was made and the patient received oral S-1 as adjuvant chemotherapy. There were no signs of disease recurrence 12 months postoperatively.
The clinical indication for conversion surgery in patients with HER2-positive AGC has not been defined or documented in the literature. Therefore, we devised a prognostic scoring system based on multivariate analysis of survival to identify suitable candidates. Age (< 70 vs. ≥ 70 years), peritoneal dissemination (P0 vs. P1), and histological type (differentiated vs. undifferentiated) were selected as important factors in our scoring system. These three pre-therapeutic factors are all well-established prognostic markers in patients with AGC [20,21,22]. However, none of the previous studies have assessed age, peritoneal dissemination, and histological type simultaneously as potential indicators for conversion surgery in patients with HER2-positive AGC after treatment with trastuzumab-based chemotherapy. This study is unique because we developed a prognostic scoring system based on three clinicopathological factors to establish the therapeutic strategy, including conversion surgery after trastuzumab-based chemotherapy, for patients with HER2-positive AGC.
The present study demonstrated a close relationship between our prognostic score and the presence or absence of conversion surgery. Among the seven patients with a score of 0, six (85.7%) underwent conversion surgery, whereas among the three patients with a score of 3, none underwent conversion surgery. Consequently, this prognostic scoring system may have clinical utility to predict the suitability of conversion surgery in the pre-therapeutic management of patients with HER2-positive AGC. Furthermore, our prognostic score correlated significantly with OS. In particular, all patients (n = 7) with a score of 0 were alive at the time of writing this article. On the other hand, patients with a score of 3 had worse prognosis than those with scores of 0, 1, or 2. These results suggest that this prognostic scoring system may be useful for predicting the prognosis associated with conversion surgery after trastuzumab-based chemotherapy for patients with HER2-positive AGC.
The present study has several limitations. First, it was a retrospective study conducted at a single institution, and the sample size was small (HER2-positive AGC patients: n = 33). Second, conversion surgery after chemotherapy was clinically indicated for patients with a PS of 0–2 and a tumor status predicted to achieve R0 curative resection based on their general condition. The treatment decision also depended on the physician’s decision. Moreover, one patient from the chemotherapy-alone group, with a complete response to trastuzumab-based chemotherapy survived for more than 5 years. Although we recommended conversion surgery, the patient declined. These limitations might have resulted in biases that could have impacted the results. Therefore, further large prospective studies are warranted to validate our findings.
In conclusion, a grading system based on age, peritoneal dissemination, and histological type has the clinical potential to predict the suitability of conversion surgery and prognosis for patients with HER2-positive AGC treated with trastuzumab-containing chemotherapy. We anticipate that our prognostic scoring system will serve as a promising tool for planning an aggressive strategy in the clinical management of patients with HER2-positive AGC.
References
Fitzmaurice C, Dicker D, Pain A, Hamavid H, Moradi-Lakeh M, Global Burden of Disease Cancer Collaboration, et al. The global burden of cancer 2013. JAMA Oncol. 2015;1:505–27.
Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA, Global Burden of Disease Cancer Collaboration, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the global burden of disease study. JAMA Oncol. 2017;3:524–48.
Bang Y-J, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–97.
Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer. 2021;24:1–21.
Yoshida K, Yamaguchi K, Okumura N, Tanahashi T, Kodera Y. Is conversion therapy possible in stage IV gastric cancer: the proposal of new biological categories of classification. Gastric Cancer. 2016;19:329–38.
Kodera Y. Surgery with curative intent for stage IV gastric cancer: is it a reality of illusion? Ann Gastroenterol Surg. 2018;2:339–47.
Arigami T, Matsushita D, Okubo K, Sasaki K, Noda M, Kita Y, et al. Clinical significance of conversion surgery for gastric cancer with peritoneal dissemination: a retrospective study. Oncology. 2020;98:798–806.
Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, Washington MK, et al. AJCC cancer staging manual. 8th ed. New York: Springer; 2017.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101–12.
Tanner M, Hollmén M, Junttila TT, Kapanen AI, Tommola S, Soini Y, et al. Amplification of HER-2 in gastric carcinoma: association with topoisomerase II alpha gene amplification, intestinal type, poor prognosis and sensitivity to trastuzumab. Ann Oncol. 2005;16:273–8.
Gravalos C, Jimeno A. HER2 in gastric cancer: a new prognostic factor and a novel therapeutic target. Ann Oncol. 2008;19:1523–9.
Hofmann M, Stoss O, Shi D, Büttner R, van de Vijver M, Kim W, et al. Assessment of a HER2 scoring system for gastric cancer: results from a validation study. Histopathology. 2008;52:797–805.
Terashima M, Kitada K, Ochiai A, Ichikawa W, Kurahashi I, Sakuramoto S, ACTS-GC Group, et al. Impact of expression of human epidermal growth factor receptors EGFR and ERBB2 on survival in stage II/III gastric cancer. Clin Cancer Res. 2012;18:5992–6000.
Seo S, Ryu M-H, Park YS, Ahn JY, Park Y, Park SR, et al. Loss of HER2 positivity after anti-HER2 chemotherapy in HER2-positive gastric cancer patients: results of the GASTric cancer HER2 reassessment study 3 (GASTHER3). Gastric Cancer. 2019;22:527–35.
Saeki H, Oki E, Kashiwada T, Arigami T, Makiyama A, Iwatsuki M, et al. Re-evaluation of HER2 status in patients with HER2-positive advanced or recurrent gastric cancer refractory to trastuzumab (KSCC1604). Eur J Cancer. 2018;105:41–9.
Beom S-H, Choi YY, Baek S-E, Li S-X, Lim JS, Son T, et al. Multidisciplinary treatment for patients with stage IV gastric cancer: the role of conversion surgery following chemotherapy. BMC Cancer. 2018;18:1116.
Hayano K, Watanabe H, Ryuzaki T, Sawada N, Ohira G, Kano M, et al. Prognostic benefit of conversion surgery for HER2 positive stage IV gastric cancer; a case series study of eleven patients treated with trastuzumab-based chemotherapy. Surg Case Rep. 2020;6:219.
Kinoshita J, Yamaguchi T, Moriyama H, Fushida S. Current status of conversion surgery for stage IV gastric cancer. Surg Today. 2021;51:1736–54.
Maehara Y, Emi Y, Baba H, Adachi Y, Akazawa K, Ichiyoshi Y, et al. Recurrences and related characteristics of gastric cancer. Br J Cancer. 1996;74:975–9.
Chau I, Norman AR, Cunningham D, Waters JS, Oates J, Ross PJ. Multivariate prognostic factor analysis in locally advanced and metastatic esophago-gastric cancer–pooled analysis from three multicenter, randomized, controlled trials using individual patient data. J Clin Oncol. 2004;22:2395–403.
Dicken BJ, Bigam DL, Cass C, Mackey JR, Joy AA, Hamilton SM. Gastric adenocarcinoma: review and considerations for future directions. Ann Surg. 2005;241:27–39.
Funding
This work was supported in part by grants-in-aid (no. 19K09200) for scientific research from the Ministry of Education, Science, Sports, and Culture, Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We have no competing interests to declare.
Ethics approval
This study was approved by the Ethics Committee of Kagoshima University (approval number: 200043).
Consent to participate
We conducted a retrospective study and used the “opt-out” method as a way to obtain informed consent from patients.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Arigami, T., Matsushita, D., Okubo, K. et al. A prognostic scoring system for conversion surgery after trastuzumab-based chemotherapy for human epidermal growth factor receptor 2-positive advanced gastric cancer. Surg Today 52, 1721–1730 (2022). https://doi.org/10.1007/s00595-022-02515-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-022-02515-6