Abstract
Background
Human epidermal growth factor receptor 2 (HER2) is likely overexpressed and/or amplified in locally advanced gastric cancer with extensive (bulky N2 or paraaortic) lymph node metastasis, and patients may benefit from treatment with anti-HER2 antibodies. This study evaluated the frequency of HER2 overexpression and amplification in The Japanese Gastric Cancer Association (JGCA)-N3 and JGCA-bulky N2 tumors and the correlation between HER2 status and survival.
Methods
HER2 status was assessed using immunohistochemistry (IHC) and fluorescence in situ hybridization (FISH) in tumor tissue samples from 89 patients with gastric adenocarcinoma enrolled in the phase II JCOG0001 and JCOG0405 trials. HER2 positivity was defined as IHC3+ or IHC2+ with confirmatory FISH results.
Results
Of the 89 tumor samples, 24 (27 %) showed HER2 positivity, including 16 scored as IHC3+ and 8 as IHC2+ and FISH positive. Multivariate analysis showed that the HER2 positivity rate was significantly higher in evaluable differentiated tumors than in undifferentiated tumors [18/44 (40.9 %) vs. 5/42 (11.9 %)]. Although the apparent OS curve of HER2 positive was superior to that of HER2 negative patients, HER2 status was not a statistically significant prognostic factor in multivariate analysis.
Conclusion
The HER2 positivity rate was relatively high in patients with JGCA-bulky N2 and JGCA-N3 gastric adenocarcinoma, suggesting that HER2 evaluation is essential to select the therapeutic regimen for neoadjuvant chemotherapy for this group of patients.
Similar content being viewed by others
Introduction
Gastric cancer is one of the most common types of malignant tumors and the second leading cause of cancer-related deaths in the world [1]. Complete tumor removal (R0 resection) is essential for cure [2, 3]. The Japanese Gastric Cancer Association (JGCA) used to define paraaortic lymph nodes (PAN) as regional lymph node stations (JGCA-N3) in contrast to the tumor node metastasis (TNM) staging of the International Union Against Cancer (UICC), which defines paraaortic metastasis as distant metastasis [4]. Prophylactic PAN dissection for T3 (subserosa) or deeper gastric cancer is no longer recommended in Japan based on the results of a randomized controlled trial (RCT) by the Japan Clinical Oncology Group (JCOG 9501) [5]. However, Japanese surgeons have not given up yet to cure patients with extensive nodal disease [bulky nodal metastasis surrounding the celiac artery and its branches (JGCA-bulky N2) or PAN metastasis] using preoperative chemotherapy with D2 plus PAN dissection (PAND) if they have no other distant metastasis. These patients are regarded as unresectable in the West and treated by palliative chemotherapy with or without radiation. The Stomach Cancer Study Group of the JCOG considers these tumors to be a specific type and has carried out two phase II studies on this subject with remarkably better results than historical controls [6, 7]. We consider that more intensive chemotherapy such as triplet therapy or the addition of molecular targeted agents is needed to further improve the prognosis of patients with this disease.
Human epidermal growth factor receptor 2 (HER2; also known as ERBB2) is a member of a family of receptors associated with tumor cell differentiation, migration, proliferation, and survival [8], and it is recognized as an important biomarker. In gastric carcinoma, the frequency of HER2 overexpression and/or amplification has been reported to vary widely from 7 to 34 % [8–11]. Trastuzumab, a monoclonal antibody targeting the extracellular domain of the HER2 protein, has been shown to confer a survival benefit in both primary and metastatic breast carcinoma cases with high levels of HER2 expression and amplification, and it is used as the standard regimen in adjuvant therapy [12–14]. Findings in a multicenter and international phase III trial to evaluate trastuzumab for gastric cancer (ToGA trial) revealed that the combination of trastuzumab with chemotherapy consisting of fluoropyrimidine and cisplatin improved survival in patients with advanced HER2-positive gastric carcinomas or gastroesophageal junction carcinomas as compared to chemotherapy alone [15]. Thus, molecular targeted therapies have begun to play an important role in improving the prognosis of patients with gastric carcinomas.
A close relationship between HER2 overexpression and/or amplification and intestinal histologic type in gastric carcinomas has been reported in recent studies and confirmed in the ToGA trial [10, 16–18]. Approximately 50 % of all patients entered in the JCOG0001 and JCOG0405 trials were pathologically diagnosed as having well-to-moderately differentiated tumors corresponding to the intestinal type. Thus, we speculated that tumors with JGCA-N3 or JGCA-bulky N2 have a high frequency of HER2 expression and/or amplification and considered it necessary to clarify whether the HER2 status of these tumors should be taken into account for development of new therapies.
The aims of this study were to evaluate the frequency of HER2 overexpression and/or amplification in tumors with JGCA-N3 or JGCA-bulky N2 using immunohistochemistry (IHC) and fluorescence in situ hybridization (FISH) findings and to analyze the correlation between HER2 status and survival.
Patients and methods
Patients and material
All gastric cancer patients enrolled in the JCOG0001 and JCOG0405 trials were included in this study [6, 7]. Eligibility criteria, treatment schedules, monitoring, and statistical analysis in these trials have been described in detail elsewhere [6, 7]. Briefly, these were phase II studies involving patients with histologically proven gastric adenocarcinoma with JGCA-N3 or JGCA-bulky N2 confirmed by contrast-enhanced computed tomography (CT) between 2000 and 2007. Eligibility criteria included no distant nodal metastasis outside the paraaortic region, as confirmed by contrast-enhanced CT, no peritoneal or pleural effusion, no clinically apparent brain or bone metastasis, no peritoneal metastasis or negative cytology obtained in staging laparoscopy, non-scirrhous macroscopic type by endoscopy or upper gastrointestinal X-ray study, and no previous chemotherapy or radiotherapy. Following preoperative chemotherapy (JCOG0001 trial: irinotecan plus cisplatin; JCOG0405 trial: S-1 plus cisplatin), a gastrectomy with D2 plus PAND was performed if curative resection was deemed possible. To examine the HER2 status of archival tumor samples surgically resected or biopsies, 3-µm-thick paraffin block samples from enrolled cases were obtained from institutions belonging to the Stomach Cancer Study Group of the JCOG and mounted on unstained glass slides. This study was separately approved by the JCOG Protocol Review Committee and the institutional review board of each participating institution as the original protocol of these studies (JCOG0001 and 0405) did not include this concept and usage of the material.
IHC and FISH tests
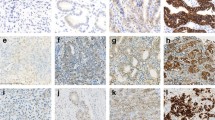
Tumors were centrally tested for HER2 status using IHC (Hercep Test, DAKO, Denmark) and FISH (HER2 FISH pharmDx, DAKO) methods. For IHC analysis, the samples were scored according to modifications of criteria originally published by Hofmann and colleagues [9], as follows: 0, no reactivity or membranous reactivity in ≤10 % of the cells; 1+, faint/barely perceptible membranous reactivity in >10 % of the cells, but cells reactive in only parts of their membranes; 2+, weak to moderate complete or basolateral membranous reactivity in >10 % of the tumor cells; 3+, moderate to strong complete or basolateral membranous reactivity in >10 % of the tumor cells. For FISH assessments, an HER2:CEP17 (centromeric probe 17) ratio ≥2 was defined as positive for HER2 amplification.
Definition of HER2-positive status
The standard criteria for HER2-positive status, including the HER2 IHC scoring system and FISH assessments, have thus far only been validated for breast cancer. However, the biological differences between breast and gastric tumors, such as tumor heterogeneity and basolateral membrane staining, were recently reported. Therefore, the ToGA trial adopted IHC3+ or FISH positivity (HER2: CEP ratio ≥2) as the definition of HER2-positive status. According to subgroup analysis in this trial, a survival benefit from the addition of trastuzumab was recognized for patients with overexpression of HER2 protein, including the IHC2+/FISH+ and IHC3+ subgroups, whereas there was no survival benefit for the IHC0/FISH+ or IHC1+/FISH+ subgroups. The European Medicine Agency has noted that trastuzumab should only be used in patients with metastatic gastric cancer whose tumors have HER2 overexpression as defined by IHC2+ and a confirmatory FISH+ result, or IHC3+ as determined by an accurate and validated assay. Therefore, we chose IHC testing as the primary method for determining HER2 status, while FISH was restricted to cases with equivocal (IHC2+) expression of HER2 protein. In this study, patients classified as IHC3+ or IHC2+/FISH+ were defined as HER2 positive. The HER2 IHC score was independently determined by three different pathologists, each belonging to a different institution: the Hyogo College of Medicine, the Research Center for Innovative Oncology in the National Cancer Center East Hospital, and the National Cancer Center Hospital. Scores were accepted if they were agreed upon by at least two of the pathologists. If a score differed among all three, the final judgment was determined by reference to FISH results in a meeting of the pathologists. Thus, we performed FISH for IHC2+ cases or those with an ambivalent score. These FISH results were also assessed by the three pathologists.
Statistical analysis
All data except for HER2 status were obtained from patient databases managed by the JCOG Data Center. Categorical and continuous data were analyzed using Fisher’s exact test and the Wilcoxon rank-sum test, respectively. Multivariate log-linear regression analysis was performed to identify factors independently associated with HER2 positivity. The HER2 positivity rate was estimated in eligible patients whose HER2 status was determined based on either biopsy or resected specimens. Survival analysis was performed in eligible patients for whom resected specimens were obtained because multivariate analysis was conducted to identify the prognostic factors at the postoperative status. The probability of survival for the different subgroups was calculated using the Kaplan-Meier method, and the significance of differences between survival curves was determined using a log-rank test. Multivariate analysis was performed using the stratified Cox’s proportional hazards model with the study as strata to identify the primary prognostic indicators independently associated with survival. All P values were two-sided and the significance level was set at P < 0.05. All analyses were carried out using SAS version 9.2 (SAS Institute, Inc., Cary, NC).
Results
Among 106 eligible patients in JCOG0001 (n = 55) and JCOG0405 (n = 51), samples of 89 patients from 22 institutions were collected and analyzed (Fig. 1). These materials comprised 86 resected stomach samples and 3 biopsy specimens as the biopsied tumors were unresectable. Sixteen samples were scored as IHC3+, and 8 that were IHC2+ were found to be FISH+. These 24 samples were diagnosed as HER2 positive, for an HER2 positivity rate of 27.0 % [95 % confidence interval (CI) 18.1–37.4 %].
Associations between clinicopathological variables and HER2 status are shown in Table 1. Univariate analysis revealed that the histology of the resected stomach, performance status (PS), and curability had a significant association with HER2 positivity. Differentiated types (papillary and tubular adenocarcinoma) showed significantly higher HER2 positivity rates than undifferentiated types (poorly differentiated adenocarcinoma, signet-ring cell carcinoma). HER2 positivity did not affect clinical or histopathological response, although there were significantly more cases of R2 resection in HER2-negative cases. In multivariate analysis, the histological type of the excised specimen was independently related to HER2 overexpression and/or amplification (Table 2). Histological examination of resected stomach samples revealed that 18 of 44 tumors of the differentiated type (40.9 %) were HER2 positive, while only 5 of 42 (11.9 %) tumors of the undifferentiated type were HER2 positive (Table 3).
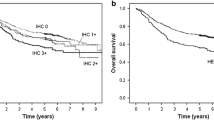
The estimated 3-year overall survival for HER2-positive cases was 66.7 % and that for HER2-negative cases was 38.7 % (p = 0.022), with a hazard ratio (HR) of 0.47 (95 % CI 0.24–0.91) (Fig. 2). The tendency of survival was almost the same in both the JCOG0001 and JCOG0405 trials, OS being always superior in Her2-positive than in -negative cases. However, in a Cox multivariate model that included age (≤64/>64), sex (male/female), clinical nodal factors (bulky N2 without PAN, bulky N2, and PAN), PS (0/1), histology of the resected stomach specimen (differentiated/undifferentiated type), and pathological response (grade 0–1a/grade 1b–3), the hazard ratio of HER2 status (positive/negative) was much higher and came close to 1.0 (HR = 0.88, P = 0.73) (Table 4). This result was almost identical to that of a multivariate analysis evaluating survival after enrollment, including pretreatment biopsy instead of resected specimen, age, sex, PS, and clinical nodal factors: the HR for HER2 status was 0.84. We also estimated the survival curve for patients with R0 resection, curability of A or B, by the Kaplan-Meier method as a sensitivity analysis. Three-year survival in Her2+ and Her2- were 65.2 % and 52.2 %, respectively. Log-rank p value was 0.32 and hazard ratio for Her2 + was 0.70 (95 % CI, 0.34-1.43). In multivariate analysis including age, sex, ECOG PS, lymph node status, histological type, and pathological grade using the study as strata, the hazard ratio for Her2+ was 1.04 (95 % CI, 0.48-2.24). Although there is a significant difference in the number of R2 patients, OS curves showed an almost similar tendency with or without exclusion of R2 cases.
As for relapse-free survival for curability A or B, the HR between HER2 positivity and negativity was 1.30 (95 % CI 0.62–2.70) in multivariate analysis, suggesting the difference between survival curves might have been due to other confounding factors.
Discussion
Although HER2 expression has come to be an indispensable factor in determining the therapeutic strategy for recurrent or unresectable advanced gastric carcinoma, the low positive rate in general still discourages clinicians from examining HER2 status before starting chemotherapy. In the present study, the HER2 positivity rate for JGCA-bulky N2 and JGCA-N3 was 27.0 %. This subgroup of gastric cancers showed higher HER2 positivity than ordinary types.
In a review of 42 studies published from 1991 to 2012, the HER2 positivity rate based on IHC scoring ranged widely from 4.4 to 53.4 % [19]. The most significant factor underlying this wide variation is likely the criteria used for determining HER2 expression, as these have not been standardized and thus differ among studies. In 2008, however, Hoffman et al. provided clear criteria based on the results of the ToGA trial, and in the subsequent 2 years the HER2 positivity rate ranged from 9.4 to 15.7 %. Thus, accuracy is now considered to be controlled to a certain degree. Meanwhile, using FISH determination, the positivity rate ranged from 8.7–18.1 %, although the dispersion of positive results was not as clear with IHC, possibly because of the lack of clear quantitative criteria in FISH. The HER2 positivity rate in the ToGA trial, if the same definition as in the present study is applied, was just 12.2 %. As the Japanese subjects in this study showed slightly higher positivity (20.0 %), the positivity rate in consecutive series of metastatic and unresectable gastric cancer with or without target lesions in Japan was studied in a prospective manner by the Japanese Foundation for Multidisciplinary Treatment of Cancer (JFMC44-1101) [20, 21]. It was, however, just 15.5 %, equivalent to that of a large Japanese study on adjuvant chemotherapy for stage II/III curatively resected patients in the ACTS-GC study (13.6 %) [11]. Of 829 subjects, 74 were scored as IHC3+ and 38 as IHC2+ and FISH+ (total positive, 113 subjects). In comparison to these results, patients with JGCA-bulky N2 or JGCA-bulky N3 are considered to constitute a subgroup showing high HER2 positivity (27.0 % in total). It has been reported that HER2 overexpression occurs more frequently in differentiated-type carcinoma or gastroesophageal junctional cancer. Generally, undifferentiated type carcinoma comprises about 60–70 % of advanced gastric carcinoma, making the differentiated type a minority. In the present study, which exclusively enrolled patients with bulky N2 or clinical metastasis to the paraaortic lymph node, the differentiated type accounted for about 50 %, which might have led to the high HER2 positivity rate.
According to a review of 42 studies published from 1991–2012, the relationship between HER2 expression and prognosis has been found to be inconsistent and remains controversial [19, 22, 23]. Accordingly, it is important to carefully interpret the findings of the present study regarding this relationship. We found a tendency toward better relapse-free survival in HER2-positive cases, and overall survival was significantly more favorable (HR = 0.47, p = 0.022) in the HER2-positive than in the HER2-negative group. However, in multivariate Cox analysis, HER2 expression was not an independent factor for survival, thus a confounding background factor is suspected. The relatively favorable prognosis in the HER2-positive group was likely affected by the lower proportion of patients who underwent R2 resection, although better OS was observed even excluding R2 patients. This might be related to the fact that while 20 % of HER2-negative patients had diffuse-type histology (por2, sig, or muc according to the Japanese classification), none of the HER2-positive patients did. It is necessary to further examine these results by conducting studies with greater numbers of subjects.
In the present study, approximately 41 % of patients with differentiated type cancer were diagnosed as HER2 positive, while the HER2-positive rate in patients with poorly differentiated type cancer was only about 12 %. The differentiated type constituted about 58 % of biopsy specimens and 51 % of resected specimens, with inconsistency observed in 7 % of cases. There are two possible explanations. First, it is well known that some undifferentiated type tumors (classified by dominance) have moderately differentiated histology in the mucosal layer and therefore are diagnosed as the differentiated type by biopsy. Another possibility is that differentiated portions of tumors were more affected by chemotherapy than undifferentiated ones, resulting in an increase in the number of tumors diagnosed as undifferentiated type defined by quantitative predominance. As HER2 status was determined based primarily on resected specimens, with biopsy specimens used in only a few patients who did not undergo gastrectomy, comparison of HER2 status before and after chemotherapy was impossible in this study. Heterogeneity of HER2 expression in a single tumor is known to be more prominent in gastric cancer than in breast cancer. Several papers, however, have reported relatively high concordance in HER2 status between biopsy specimens and resected material [24–26]. The limited information available in this study hampers further discussion of the effects of chemotherapy in relation to tumor differentiation and HER2 status.
While it is known that overfixation with formalin affects immunostaining, it was previously reported that no difference was observed in HER2 staining intensity between samples with 120 h of fixation and those with 3 h of fixation [27]. It was also shown that when the time from sample collection to fixation exceeded 2 h, signals related to HER2 expression became weak, significantly affecting FISH determination. Furthermore, in IHC determination, intensity is likely to decrease when duration of fixation approaches 1 week. In a previous study of breast carcinoma, scores in the IHC3+ group were not affected even after formalin fixation times over 2 h, while in the FISH group, the peripheral cellular borders became indefinite, FISH signals decreased, and nuclear resolution was reduced [28]. Another report noted that the retention time in a paraffin block might affect IHC or FISH determination of HER2 expression [29]. In the present study, we used specimens collected from subjects who had been registered for two different phase II trials conducted in multiple institutions before the results of the ToGA study were reported. Since fixation method and time were not standardized in these trials, it is possible that variations in these factors might have affected expression. Moreover, tumor degeneration almost certainly influenced the effects of chemotherapy on immunostaining and FISH results, as the specimens were collected after preoperative chemotherapy. Because of the high heterogeneity of gastric cancer, IHC diagnostic criteria for HER2 overexpression in surgically resected materials differ from those in prior biopsy specimens [9]. When diagnostic criteria were used, the concordance of IHC-based HER2 scoring between surgically resected materials and prior biopsy specimens with an HER2 score of 3+ was reported to be high, and at least three or four fragments seemed sufficient for assessing IHC HER2 status based on biopsy material [30]. In the present study, in fact, the difference in the hazard ratio for overall survival of HER2-positive patients after chemotherapy in two analyses comparing differentiated and undifferentiated types using pretreatment histology based on biopsy or resected specimens was small (0.84 and 0.88). However, further validation of equivalence is needed because of the increasingly frequent use of preoperative chemotherapy, as neoadjuvant treatment is regarded as essential in Japan for patients with JGCA-bulky N2 or JGCA-N3, while they are regarded incurable in the West. Selection of the chemotherapy regimen in neoadjuvant treatment is of paramount importance for these patients.
In conclusion, our results demonstrated that patients with JGCA-bulky N2 or JGCA-N3 constituted a subgroup with gastric cancer marked by a high HER2 positivity rate and may be a target population for trastuzumab administration. Presently, a phase II trial with a preoperative triplet chemotherapy (S-1 + cisplatin + docetaxel) regimen is underway for this subgroup of gastric cancer patients in Japan. Nevertheless, it is necessary to conduct clinical studies to determine whether better prognosis in HER2-positive patients can be attained with multidrug therapy, including trastuzumab. This will aid in establishing treatment development pathways based on the presence of HER2 expression, which is currently the only reliable biomarker in gastric cancer.
References
Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24:2137–50.
Sasako M. Principles of surgical treatment for curable gastric cancer. J Clin Oncol. 2003;21(23 Suppl):274s–5s.
Dickson JL, Cunningham D. Systemic treatment of gastric cancer. Eur J Gastroenterol Hepatol. 2004;16:255–63.
Japanese Gastric Cancer. A. Japanese Classification of Gastric Carcinoma, 2nd English Edition. Gastric Cancer. 1998;1:10–24.
Sasako M, Sano T, Yamamoto S, Kurokawa Y, Nashimoto A, Kurita A, et al. D2 lymphadenectomy alone or with para-aortic nodal dissection for gastric cancer. N Engl J Med. 2008;359:453–62.
Yoshikawa T, Sasako M, Yamamoto S, Sano T, Imamura H, Fujitani K, et al. Phase II study of neoadjuvant chemotherapy and extended surgery for locally advanced gastric cancer. Br J Surg. 2009;96:1015–22.
Tsuburaya A, Mizusawa J, Tanaka Y, Fukushima N, Nashimoto A, Sasako M. Neoadjuvant chemotherapy with S-1 and cisplatin followed by D2 gastrectomy with para-aortic lymph node dissection for gastric cancer with extensive lymph node metastasis. Br J Surg. 2014;101:653–60.
Gravalos C, Jimeno A. HER2 in gastric cancer: a new prognostic factor and a novel therapeutic target. Ann Oncol. 2008;19:1523–9.
Hofmann M, Stoss O, Shi D, Buttner R, van de Vijver M, Kim W, et al. Assessment of a HER2 scoring system for gastric cancer: results from a validation study. Histopathology. 2008;52:797–805.
Tanner M, Hollmen M, Junttila TT, Kapanen AI, Tommola S, Soini Y, et al. Amplification of HER-2 in gastric carcinoma: association with Topoisomerase IIalpha gene amplification, intestinal type, poor prognosis and sensitivity to trastuzumab. Ann Oncol. 2005;16:273–8.
Terashima M, Kitada K, Ochiai A, Ichikawa W, Kurahashi I, Sakuramoto S, et al. Impact of expression of human epidermal growth factor receptors EGFR and ERBB2 on survival in stage II/III gastric cancer. Clin Cancer Res. 2012;18:5992–6000.
Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–92.
Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–72.
Smith I, Procter M, Gelber RD, Guillaume S, Feyereislova A, Dowsett M, et al. 2-year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: a randomised controlled trial. Lancet. 2007;369:29–36.
Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–97.
Grabsch H, Sivakumar S, Gray S, Gabbert HE, Muller W. HER2 expression in gastric cancer: rare, heterogeneous and of no prognostic value - conclusions from 924 cases of two independent series. Cell Oncol. 2010;32:57–65.
Barros-Silva JD, Leitao D, Afonso L, Vieira J, Dinis-Ribeiro M, Fragoso M, et al. Association of ERBB2 gene status with histopathological parameters and disease-specific survival in gastric carcinoma patients. Br J Cancer. 2009;100:487–93.
Park DI, Yun JW, Park JH, Oh SJ, Kim HJ, Cho YK, et al. HER-2/neu amplification is an independent prognostic factor in gastric cancer. Dig Dis Sci. 2006;51:1371–9.
Jorgensen JT, Hersom M. HER2 as a Prognostic Marker in Gastric Cancer - A Systematic Analysis of Data from the Literature. J Cancer. 2012;3:137–44.
Sawaki A, Ohashi Y, Omuro Y, Satoh T, Hamamoto Y, Boku N, et al. Efficacy of trastuzumab in Japanese patients with HER2-positive advanced gastric or gastroesophageal junction cancer: a subgroup analysis of the Trastuzumab for Gastric Cancer (ToGA) study. Gastric Cancer. 2012;15:313–22.
Nishikawa K, Chin K, Nashimoto A, Miki A, Miwa H, Tsuburaya A, et al. Result of HER2 status in Japanese metastatic gastric cancer: Prospective cohort study (JFMC44-1101). J Clin Oncol. 2013;31(suppl 4; abstr 10).
Yoon HH, Shi Q, Sukov WR, Lewis MA, Sattler CA, Wiktor AE, et al. Adverse prognostic impact of intratumor heterogeneous HER2 gene amplification in patients with esophageal adenocarcinoma. J Clin Oncol. 2012;30:3932–8.
Okines AF, Thompson LC, Cunningham D, Wotherspoon A, Reis-Filho JS, Langley RE, et al. Effect of HER2 on prognosis and benefit from peri-operative chemotherapy in early oesophago-gastric adenocarcinoma in the MAGIC trial. Ann Oncol. 2013;24:1253–61.
Pirrelli M, Caruso ML, Di Maggio M, Armentano R, Valentini AM. Are biopsy specimens predictive of HER2 status in gastric cancer patients? Dig Dis Sci. 2013;58:397–404.
Lee S, de Boer WB, Fermoyle S, Platten M, Kumarasinghe MP. Human epidermal growth factor receptor 2 testing in gastric carcinoma: issues related to heterogeneity in biopsies and resections. Histopathology. 2011;59:832–40.
Yano T, Doi T, Ohtsu A, Boku N, Hashizume K, Nakanishi M, et al. Comparison of HER2 gene amplification assessed by fluorescence in situ hybridization and HER2 protein expression assessed by immunohistochemistry in gastric cancer. Oncol Rep. 2006;15: 65-71.
Ibarra JA, Rogers LW. Fixation time does not affect expression of HER2/neu: a pilot study. Am J Clin Pathol. 2010;134:594–6.
Khoury T, Sait S, Hwang H, Chandrasekhar R, Wilding G, Tan D, et al. Delay to formalin fixation effect on breast biomarkers. Mod Pathol. 2009;22:1457–67.
Risio M, De Rosa G, Sarotto I, Casorzo L, Capussotti L, Torchio B, et al. HER2 testing in gastric cancer: molecular morphology and storage time-related changes in archival samples. Int J Oncol. 2003;23:1381–7.
Shinozaki E, Yamamoto N, Chin K, Ogura M, Suenaga M, Matsusaka S, et al. How many biopsy fragments will be necessary to assess HER2 status for gastric cancer?. J Clin Oncol, 2012 Gastrointestinal Cancers Symposium. Vol 30, No 4_suppl (February 1 Supplement), 2012: 40.
Acknowledgments
This study was supported by the National Cancer Center Research and Development Fund (23-A-16, 23-A-19). The authors thank the members of the JCOG Data Center and Operations Office for their support, especially to Dr. K. Nakamura and Dr. K. Kataoka, for preparation of the manuscript and Dr. H. Fukuda for oversight of study management.
Conflict of interest
Dr. Mitsuru Sasako received lecture fees from Taiho Pharmaceutical Co., Ltd., and Chugai Pharmaceutical Co., Ltd. Dr. Atsushi Ochiai received lecture fees from Chugai. The institution of Dr. Tomohiro Matsumoto and Dr. Mitsuru Sasako received research grants from Taiho and Chugai. The institution of Dr. Atsushi Ochiai received research grants from Taiho, Merck Serono Co., Ltd., Bayer Yakuhin, Ltd., and Amgen Inc. The other authors report no conflict of interest.
Author information
Authors and Affiliations
Consortia
Corresponding author
Appendix
Appendix
Investigators in participating institutions: Tokyo Metropolitan Cancer and Infectious Diseases Center Komagome Hospital, Y. Iwasaki; Sakai Municipal Hospital, H. Furukawa; Gifu Municipal Hospital, H. Oshita; Aichi Cancer Center Research Institute, S. Ito; Iwate Medical University School of Medicine, K. Koeda; Miyagi Cancer Center, T. Fujiya; Osaka National Hospital, T. Tsujinaka; Osaka Medical College, H. Takiuchi; National Hospital Organization Shikoku Cancer Center, A. Kurita; National Defense Medical College, K. Hase; National Cancer Center Hospital East, T. Kinoshita; Tokyo Metropolitan Bokuto Hospital, S. Inoue; Fujita Health University School of Medicine, I. Uyama; National Hospital Organization Sendai Medical Center, T. Saito; Tsubame Rosai Hospital, K. Miyashita; Wakayama Medical University School of Medicine, H. Yamaue; Hiroshima City Hospital, M. Ninomiya
Rights and permissions
About this article
Cite this article
Matsumoto, T., Sasako, M., Mizusawa, J. et al. HER2 expression in locally advanced gastric cancer with extensive lymph node (bulky N2 or paraaortic) metastasis (JCOG1005-A trial). Gastric Cancer 18, 467–475 (2015). https://doi.org/10.1007/s10120-014-0398-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10120-014-0398-3