Abstract
Purposes
Serum P53 antibody (S-P53Ab) is reportedly an effective screening tool for cancer. The aim of this study is to investigate the clinical significance of tumor markers combination in colorectal cancer (CRC) patients.
Methods
Carcinoembryonic antigen (CEA), carbohydrate 19 - 9 (CA19-9), and S-P53Ab levels were measured before tumor resection. Of the CRC patients with primary tumor resection at Kumamoto University Hospital, a total of 244 with available preoperative data for these three tumor markers were eligible for this study. The associations of the tumor markers with clinicopathological factors and the prognosis were examined using univariate and multivariate analyses.
Results
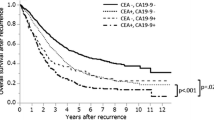
S-P53Ab positivity was strongly correlated with rectal cancer, depth of tumor invasion, lymph node metastasis, and lymphatic invasion. The ratio of S-P53Ab positivity was higher than that of CEA or CA19-9 in patients with stage 0/I disease. S-P53Ab had no power to predict the prognosis (P = 0.786). The patients with combined CEA and CA19-9 positivity had a significantly poorer overall survival than those with positivity for neither or only one, and combined CEA and CA19-9 positivity was an exclusive independent prognostic factor (P = 0.034).
Conclusions
The clinical significance of S-P53Ab measurement in CRC patients is limited. However, the combination of CEA and CA19-9 levels may be effective for predicting the outcomes of CRC treatment.


Similar content being viewed by others
References
Ferrone CR, Finkelstein DM, Thayer SP, Muzikansky A, Fernandez-delCastillo C, Warshaw AL. Perioperative CA19-9 levels can predict stage and survival in patients with resectable pancreatic adenocarcinoma. J Clin Oncol. 2006;24:2897–902.
Thirunavukarasu P, Sukumar S, Sathaiah M, Mahan M, Pragatheeshwar KD, Pingpank JF, et al. C-stage in colon cancer: implications of carcinoembryonic antigen biomarker in staging, prognosis, and management. J Natl Cancer Inst. 2011;103:689–97.
Shimada H, Noie T, Ohashi M, Oba K, Takahashi Y. Clinical significance of serum tumor markers for gastric cancer: a systematic review of literature by the Task Force of the Japanese Gastric Cancer Association. Gastric Cancer. 2014;17:26–33.
Shimada Y, Kido T, Kameyama H, Nakano M, Yagi R, Tajima Y, et al. Clinical significance of perineural invasion diagnosed by immunohistochemistry with anti-S100 antibody in Stage I-III colorectal cancer. Surg Today. 2015;45:1493–500.
Katayama H, Kurokawa Y, Nakamura K, Ito H, Kanemitsu Y, Masuda N, et al. Extended Clavien-Dindo classification of surgical complications: Japan Clinical Oncology Group postoperative complications criteria. Surg Today. 2016;46:668–85.
Stiksma J, Grootendorst DC, van der Linden PW. CA 19-9 As a Marker in Addition to CEA to Monitor Colorectal Cancer. Clin Colorectal Cancer. 2014;13:239–44.
Duffy MJ, Synnott NC, McGowan PM, Crown J, O’Connor D, Gallagher WM. p53 as a target for the treatment of cancer. Cancer Treat Rev. 2014;40:1153–60.
Suppiah A, Greenman J. Clinical utility of anti-p53 auto-antibody: systematic review and focus on colorectal cancer. World J Gastroenterol. 2013;19:4651–70.
Soussi T. p53 Antibodies in the sera of patients with various types of cancer: a review. Cancer Res. 2000;60:1777–88.
Ochiai H, Ohishi T, Osumi K, Tokuyama J, Urakami H, Seki S, et al. Reevaluation of serum p53 antibody as a tumor marker in colorectal cancer patients. Surg Today. 2012;42:164–8.
Yamaguchi T, Takii Y, Maruyama S. Usefulness of serum p53 antibody measurement in colorectal cancer: an examination of 1384 primary colorectal cancer patients. Surg Today. 2014;44:1529–35.
Sobin LH GM, Wittekind C. TNM classification of malignant tumours. 7th ed. New York: Wiley-Blackwell; 2009.
The Japanese Society for Cancer of the Colon and Rectum. JSCCR guidelines 2005 for the treatment of colorectal cancer. Tokyo: Kanehara & Co, Ltd; 2005.
The Japanese Society for Cancer of the Colon and Rectum. JSCCR guidelines 2010 for the treatment of colorectal cancer. Tokyo: Kanehara & Co, Ltd; 2010.
Watanabe T, Itabashi M, Shimada Y, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2010 for the treatment of colorectal cancer. Int J Clin Oncol 2010. 2012;17(1):21–29.
Chang SC, Lin JK, Lin TC, Liang WY. Genetic alteration of p53, but not overexpression of intratumoral p53 protein, or serum p53 antibody is a prognostic factor in sporadic colorectal adenocarcinoma. Int J Oncol. 2005;26:65–75.
Lechpammer M, Lukac J, Lechpammer S, Kovacevic D, Loda M, Kusic Z. Humoral immune response to p53 correlates with clinical course in colorectal cancer patients during adjuvant chemotherapy. Int J Colorectal Dis. 2004;19:114–120.
Shimada H, Ochiai T, Nomura F. Titration of serum p53 antibodies in 1,085 patients with various types of malignant tumors: a multiinstitutional analysis by the Japan p53 Antibody Research Group. Cancer. 2003;97:682–689.
Angelopoulou K, Stratis M, Diamandis EP. Humoral immune response against p53 protein in patients with colorectal carcinoma. Int J Cancer. 1997;70:46–51.
Hata K, Yamamoto Y, Kiyomatsu T, Tanaka T, Kazama S, Nozawa H, et al. Hereditary gastrointestinal cancer. Surg Today. 2016;46:1115–1122.
Goel A, Boland CR. Epigenetics of colorectal cancer. Gastroenterology. 2012;143:1442–1460.
Tang R, Ko MC, Wang JY, Changchien CR, Chen HH, Chen JS, et al. Humoral response to p53 in human colorectal tumors: a prospective study of 1209 patients. Int J Cancer. 2001;94:859–863.
Suppiah A, Alabi A, Madden L, Hartley JE, Monson JR, Greenman J. Anti-p53 autoantibody in colorectal cancer: prognostic significance in long-term follow-up. Int J Colorectal Dis. 2008;23:595–600.
Hammel P, Leroy-Viard K, Chaumette MT, Villaudy J, Falzone MC, Rouillard D, et al. Correlations between p53-protein accumulation, serum antibodies and gene mutation in colorectal cancer. Int J Cancer. 1999;81:712–718.
Slattery ML, Curtin K, Wolff RK, Boucher KM, Sweeney C, Edwards S, et al. A comparison of colon and rectal somatic DNA alterations. Dis Colon Rectum. 2009;52:1304–1311.
Lopes A, Bezerra AL, Pinto CA, Serrano SV, de Mell OC, Villa LL. p53 as a new prognostic factor for lymph node metastasis in penile carcinoma: analysis of 82 patients treated with amputation and bilateral lymphadenectomy. J Urol. 2002;168:81–86.
Han U, Can OI, Han S, Kayhan B, Onal BU. Expressions of p53, VEGF C, p21: could they be used in preoperative evaluation of lymph node metastasis of esophageal squamous cell carcinoma? Dis Esophagus. 2007;20:379–385.
Otto N, Schulz P, Scholz A, Hauff P, Schlegelberger B, Detjen KM, et al. The proline TP53 variant stimulates likely lymphangiogenesis in an orthotopic mouse model of pancreatic cancer. Br J Cancer. 2012;106:348–357.
Duffy MJ, van Dalen A, Haglund C, Hansson L, Klapdor R, Lamerz R, et al. Clinical utility of biochemical markers in colorectal cancer: European Group on Tumour Markers (EGTM) guidelines. Eur J Cancer. 2003;39:718–727.
Hanke B, Riedel C, Lampert S, Happich K, Martus P, Parsch H, et al. CEA and CA 19-9 measurement as a monitoring parameter in metastatic colorectal cancer (CRC) under palliative first-line chemotherapy with weekly 24-hour infusion of high-dose 5-fluorouracil (5-FU) and folinic acid (FA). Ann Oncol. 2001;12:221–226.
Narimatsu H, Iwasaki H, Nakayama F, Ikehara Y, Kudo T, Nishihara S, et al. Lewis and secretor gene dosages affect CA19-9 and DU-PAN-2 serum levels in normal individuals and colorectal cancer patients. Cancer Res. 1998;58:512–518.
La Greca G, Sofia M, Lombardo R, Latteri S, Ricotta A, Puleo S, et al. Adjusting CA19-9 values to predict malignancy in obstructive jaundice: influence of bilirubin and C-reactive protein. World J Gastroenterol. 2012;18:4150–4155.
Mandel JS, Bond JH, Church TR, Snover DC, Bradley GM, Schuman LM, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control Study. N Engl J Med. 1993;328:1365–1371.
Lieberman DA, Weiss DG. One-time screening for colorectal cancer with combined fecal occult-blood testing and examination of the distal colon. N Engl J Med. 2001;345:555–560.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Ryuma Tokunaga and other co-authors have no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tokunaga, R., Sakamoto, Y., Nakagawa, S. et al. The utility of tumor marker combination, including serum P53 antibody, in colorectal cancer treatment. Surg Today 47, 636–642 (2017). https://doi.org/10.1007/s00595-016-1464-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-016-1464-8