Abstract
Background
The serum carcinoembryonic antigen (CEA) test is mainly used for postoperative surveillance of colorectal cancer patients in Western and Japanese guidelines, but evidence to support the use of CA19-9 is scarce.
Methods
We analyzed the cohort data from 22 institutions of the Japanese Study Group for Postoperative Follow-up of Colorectal Cancer. Patients who had undergone curative surgery for primary colorectal cancer (pathological stage I–III) between 1997 and 2006 were eligible for analysis. Sensitivities of CEA and CA19-9 at the time of recurrence and the contribution of CA19-9 to detecting recurrences were assessed.
Results
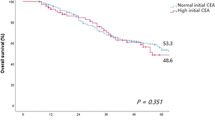
A total of 17,833 patients were eligible, and the overall recurrence rate was 18 %. The sensitivity of CA19-9 in detecting recurrence was lower than that of CEA (29 vs. 57 %). Among patients with recurrence, recurrences were first suspected in 96 % using standard surveillance modalities (CEA elevation, CT scan, clinic visit, and colonoscopy), whereas recurrences were suspected because of CA19-9 elevation in an estimated 1.3 % of patients. With regard to prognosis after recurrences, the sensitivity of CA19-9 was lower than that of CEA in the detection of surgically treatable recurrences (22 vs. 49 %). In terms of overall survival after recurrences, CA19-9 and CEA had almost comparable hazard ratios (1.66 and 1.48, respectively).
Conclusions
Our data suggested that the sensitivity of serum CA19-9 test is low, and that adding it to the current standard surveillance strategies is not beneficial.

Similar content being viewed by others
References
Renehan AG, Egger M, Saunders MP et al (2002) Impact on survival of intensive follow up after curative resection for colorectal cancer: systematic review and meta-analysis of randomised trials. BMJ 324:813
Figueredo A, Rumble RB, Maroun J et al (2003) Follow-up of patients with curatively resected colorectal cancer: a practice guideline. BMC Cancer 3:26
Renehan AG, Egger M, Saunders MP et al (2005) Mechanisms of improved survival from intensive followup in colorectal cancer: a hypothesis. Br J Cancer 92:430–433
M Jeffery, BE Hickey, PN Hider (2007) Follow-up strategies for patients treated for non-metastatic colorectal cancer. Cochrane Database Syst Rev (1):CD002200
Tjandra JJ, Chan MK (2007) Follow-up after curative resection of colorectal cancer: a meta-analysis. Dis Colon Rectum 50:1783–1799
Watanabe T, Itabashi M, Shimada Y et al (2015) Japanese Society for Cancer of the Colon and Rectum (JSCCR) Guidelines 2014 for treatment of colorectal cancer. Int J Clin Oncol 20:207–239
Sears HF, Herlyn M, Del Villano B et al (1982) Monoclonal antibody detection of a circulating tumor-associated antigen. II. A longitudinal evaluation of patients with colorectal cancer. J Clin Immunol 2:141–149
Szymendera JJ, Nowacki MP, Kozlowicz-Gudzinska I et al (1985) Value of serum levels of carcinoembryonic antigen, CEA, and gastrointestinal cancer antigen, GICA or CA 19-9, for preoperative staging and postoperative monitoring of patients with colorectal carcinoma. Dis Colon Rectum 28:895–899
Novis BH, Gluck E, Thomas P et al (1986) Serial levels of CA 19-9 and CEA in colonic cancer. J Clin Oncol 4:987–993
Filella X, Molina R, Grau JJ et al (1992) Prognostic value of CA 19.9 levels in colorectal cancer. Ann Surg 216:55–59
Nicolini A, Caciagli M, Zampieri F et al (1995) Usefulness of CEA, TPA, GICA, CA 72.4, and CA 195 in the diagnosis of primary colorectal cancer and at its relapse. Cancer Detect Prev 19:183–195
Plebani M, De Paoli M, Basso D et al (1996) Serum tumor markers in colorectal cancer staging, grading, and follow-up. J Surg Oncol 62:239–244
Nakayama T, Watanabe M, Teramoto T et al (1997) Slope analysis of CA19-9 and CEA for predicting recurrence in colorectal cancer patients. Anticancer Res 17:1379–1382
Griesenberg D, Nurnberg R, Bahlo M et al (1999) CEA, TPS, CA 19-9 and CA 72-4 and the fecal occult blood test in the preoperative diagnosis and follow-up after resective surgery of colorectal cancer. Anticancer Res 19:2443–2450
Holubec L Jr, Topolcan O, Pikner R et al (2000) The significance of CEA, CA19-9 and CA72-4 in the detection of colorectal carcinoma recurrence. Anticancer Res 20:5237–5244
Franchi F, Pastore C, Izzo P et al (2001) Ca 19-9 in the monitoring of colorectal cancer after surgery. Med Oncol 18:237–238
Spila A, Ferroni P, Cosimelli M et al (2001) Comparative analysis of CA 242 and CA 19-9 serum tumor markers in colorectal cancer patients. A longitudinal evaluation. Anticancer Res 21:1263–1270
Morita S, Nomura T, Fukushima Y et al (2004) Does serum CA19-9 play a practical role in the management of patients with colorectal cancer? Dis Colon Rectum 47:227–232
Yang SH, Jiang JK, Chang SC et al (2013) Clinical significance of CA19-9 in the follow-up of colorectal cancer patients with elevated preoperative serum CA19-9. Hepatogastroenterology 60:1021–1027
Yakabe T, Nakafusa Y, Sumi K et al (2010) Clinical significance of CEA and CA19-9 in postoperative follow-up of colorectal cancer. Ann Surg Oncol 17:2349–2356
Adam R, Delvart V, Pascal G et al (2004) Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: a model to predict long-term survival. Ann Surg 240:644–657 (discussion 657–658)
Beppu T, Sakamoto Y, Hasegawa K et al (2012) A nomogram predicting disease-free survival in patients with colorectal liver metastases treated with hepatic resection: multicenter data collection as a Project Study for Hepatic Surgery of the Japanese Society of Hepato-Biliary-Pancreatic Surgery. J Hepatobiliary Pancreat Sci 19:72–84
Sasaki A, Kawano K, Inomata M et al (2005) Value of serum carbohydrate antigen 19-9 for predicting extrahepatic metastasis in patients with liver metastasis from colorectal carcinoma. Hepatogastroenterology 52:1814–1819
Primrose JN, Perera R, Gray A et al (2014) Effect of 3–5 years of scheduled CEA and CT follow-up to detect recurrence of colorectal cancer: the FACS randomized clinical trial. JAMA 311:263–270
Jones RP, McWhirter D, Fretwell VL et al (2015) Clinical follow-up does not improve survival after resection of stage I–III colorectal cancer: a cohort study. Int J Surg 17:67–71
Acknowledgments
The authors thank all members of the institutions belonging to the Japanese Study Group for Postoperative Follow-up of Colorectal Cancer who participated in this study: Ichiro Takemasa (Sapporo Medical University); Kenichi Hakamada (Hirosaki University); Hitoshi Kameyama (Niigata University); Yasumasa Takii (Niigata Cancer Center Hospital); Yoshiki Kajiwara and Kazuo Hase (National Defense Medical College); Kenjiro Kotake (Tochigi Cancer Center); Toshiaki Watanabe (Tokyo University); Keiichi Takahashi (Tokyo Metropolitan Cancer and Infectious Diseases Center Komagome Hospital); Yukihide Kanemitsu (National Cancer Center Hospital); Michio Itabashi (Tokyo Women’s Medical University); Hidero Yano (National Center for Global Health and Medicine); Shinichi Yamauchi and Masamichi Yasuno (Tokyo Medical and Dental University); Hirotoshi Hasegawa (Keio University); Yoichiro Hashiguchi (Teikyo University); Tadahiko Masaki (Kyorin University); Masahiko Watanabe (Kitasato University); Kotaro Maeda (Fujita Health University); Kouji Komori (Aichi Cancer Center Hospital); Masayuki Ohue (Osaka Medical Center for Cancer and Cardiovascular Diseases); Naohiro Tomita (Hyogo College of Medicine); and Yoshito Akagi (Kurume University).
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Okamura, R., Hasegawa, S., Hida, K. et al. The role of periodic serum CA19-9 test in surveillance after colorectal cancer surgery. Int J Clin Oncol 22, 96–101 (2017). https://doi.org/10.1007/s10147-016-1027-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-016-1027-4