Abstract
Purpose
The aim of the present study was to determine the accuracy of yearly postoperative monitoring of serum tumor markers to either detect or rule out recurrence in colorectal cancer patients.
Methods
A total of 127 colorectal cancer patients who underwent curative surgery were enrolled. The serum carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9) levels were assayed, and radiological examinations were performed routinely for 5 years after surgery or until recurrence was detected. Yearly recurrence rates (number of recurrences/number of patients assessed in a given year), sensitivities, specificities, and likelihood ratios were calculated. Post-test probabilities were calculated from these values.
Results
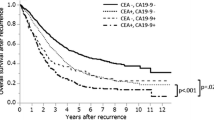
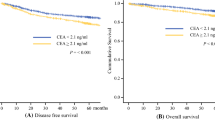
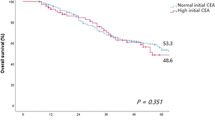
Recurrences tended to show almost the same frequencies in the first and second year after surgery (20 of 127 patients and 18 of 107 patients, respectively). However, the post-test probability of recurrence in patients with positive and negative serum CEA levels was significantly lower in the second year than in the first year (test positive: 40.0% and 76.0%; test negative: 9.3% and 0.5%, respectively).
Conclusions
Measuring CEA can help to identify patients likely to demonstrate recurrence with high accuracy only within the first year after surgery. Another examination, such as imaging, is therefore necessary for monitoring patients at 2 or more years after surgery.
Similar content being viewed by others
References
Rockall TA, McDonald PJ. Carcinoembryonic antigen: its value in the follow-up of patients with colorectal cancer. Int J Colorectal Dis 1999;13:73–77.
Mariani G, Carmellini M, Bonaguidi F, Benelli MA, Toni MG. Serum CEA monitoring in the follow-up of colorectal cancer patients with negative preoperative serum CEA. Eur J Cancer 1980;16:1099–1103.
Desch CE, Benson AB 3rd, Somerfield MR, Flynn PJ, Krause C, Loprinzi CL, et al. Colorectal cancer surveillance: 2005 update of an American Society of Clinical Oncology practice guideline. J Clin Oncol 2005;23:8512–8519.
Locker GY, Hamilton S, Harris J, Jessup JM, Kemeny N, Macdonald JS, et al. ASCO 2006 update of recommendations for the use of tumor markers in gastrointestinal cancer. J Clin Oncol 2006;24:5313–5327.
Tveit KM. ESMO minimum clinical recommendations for diagnosis, treatment and follow-up of rectal cancer. Ann Oncol 2003;14:1006–1007.
Duffy MJ, van Dalen A, Haglund C, Hansson L, Klapdor R, Lamerz R, et al. Clinical utility of biochemical markers in colorectal cancer: European Group on Tumour Markers (EGTM) guidelines. Eur J Cancer 2003;39:718–727.
Nakayama T, Watanabe M, Teramoto T, Kitajima M. CA19-9 as a predictor of recurrence in patients with colorectal cancer. J Surg Oncol 1997;66:238–243.
Reiter W, Stieber P, Reuter C, Nagel D, Lau-Werner U, Lamerz R. Multivariate analysis of the prognostic value of CEA and CA 19-9 serum levels in colorectal cancer. Anticancer Res 2000;20:5195–5198.
Filella X, Molina R, Pique JM, Garcia-Valdecasas JC, Grau JJ, Novell F, et al. Use of CA 19-9 in the early detection of recurrences in colorectal cancer: comparison with CEA. Tumour Biol 1994;15:1–6.
Duffy MJ, van Dalen A, Haglund C, Hansson L, Holinski-Feder E, Klapdor R, et al. Tumour markers in colorectal cancer: European Group on Tumour Markers (EGTM) guidelines for clinical use. Eur J Cancer 2007;43:1348–1360.
Duffy MJ. Carcinoembryonic antigen as a marker for colorectal cancer: is it clinically useful? Clin Chem 2001;47:624–630.
Korner H, Soreide K, Stokkeland PJ, Soreide JA. Diagnostic accuracy of serum-carcinoembryonic antigen in recurrent colorectal cancer: a receiver operating characteristic curve analysis. Ann Surg Oncol 2007;14:417–423.
Bhandari M, Guyatt GH. How to appraise a diagnostic test. World J Surg 2005;29:561–566.
Wichmann MW, Lau-Werner U, Muller C, Hornung HM, Stieber P, Schildberg FW. Carcinoembryonic antigen for the detection of recurrent disease following curative resection of colorectal cancer. Anticancer Res 2000;20:4953–4955.
Fernandes LC, Kim SB, Saad SS, Matos D. Value of carcinoembryonic antigen and cytokeratins for the detection of recurrent disease following curative resection of colorectal cancer. World J Gastroenterol 2006;12:3891–3894.
De Salvo L, Razzetta F, Arezzo A, Tassone U, Bogliolo G, Bruzzone D, et al. Surveillance after colorectal cancer surgery. Eur J Surg Oncol 1997;23:522–525.
Moertel CG, Schutt AJ, Go VL. Carcinoembryonic antigen test for recurrent colorectal carcinoma. Inadequacy for early detection. JAMA 1978;239:1065–1066.
Clinical practice guidelines for the use of tumor markers in breast and colorectal cancer. Adopted on May 17, 1996 by the American Society of Clinical Oncology. J Clin Oncol 1996;14:2843–2877.
Hara M, Kanemitsu Y, Hirai T, Komori K, Kato T. Negative serum carcinoembryonic antigen has insufficient accuracy for excluding recurrence from patients with Dukes C colorectal cancer: analysis with likelihood ratio and posttest probability in a follow-up study. Dis Colon Rectum 2008;11:1675–1680.
Sackett DL, Strauss SE, Richardson WS. Evidence-based medicine: how to practice and teach EBM. New York: Churchill Livingstone; 2000.
Akobeng AK. Understanding diagnostic tests 2: likelihood ratios, pre- and post-test probabilities and their use in clinical practice. Acta Paediatr 2007;96:487–491.
Davidson M. The interpretation of diagnostic test: a primer for physiotherapists. Aust J Physiother 2002;48:227–232.
Welch JP, Donaldson GA. Detection and treatment of recurrent cancer of the colon and rectum. Am J Surg 1978;135:505–511.
Sadahiro S, Suzuki T, Ishikawa K, Nakamura T, Tanaka Y, Masuda T, et al. Recurrence patterns after curative resection of colorectal cancer in patients followed for a minimum of ten years. Hepatogastroenterology 2003;50:1362–1366.
Moerte, CG, Fleming TR, Macdonald JS, Haller DG, Laurie JA, Tangen C. An evaluation of the carcinoembryonic antigen (CEA) test for monitoring patients with resected colon cancer JAMA 1993;270:943–947.
McCall JL, Black RB, Rich CA, Harvey JR, Baker RA, Watts J, et al. The value of serum carcinoembryonic antigen in predicting recurrent disease following curative resection of colorectal cancer. Dis Colon Rectum 1994;37:875–881.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hara, M., Sato, M., Takahashi, H. et al. Accuracy of monitoring serum carcinoembryonic antigen levels in postoperative stage III colorectal cancer patients is limited to only the first postoperative year. Surg Today 41, 1357–1362 (2011). https://doi.org/10.1007/s00595-010-4519-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-010-4519-2