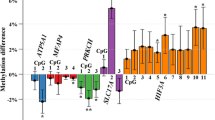
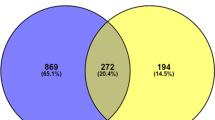
Abstract
Gestational diabetes mellitus (GDM) is a common metabolic disorder, usually diagnosed during the third trimester of pregnancy that usually disappears after delivery. In GDM, the excess of glucose, fatty acids, and amino acids results in foetuses large for gestational age. Hyperglycaemia and insulin resistance accelerate the metabolism, raising the oxygen demand, and creating chronic hypoxia and inflammation. Women who experienced GDM and their offspring are at risk of developing type-2 diabetes, obesity, and other metabolic or cardiovascular conditions later in life. Genetic factors may predispose the development of GDM; however, they do not account for all GDM cases; lifestyle and diet also play important roles in GDM development by modulating epigenetic signatures and the body’s microbial composition; therefore, this is a condition with a complex, multifactorial aetiology. In this context, we revised published reports describing GDM-associated single-nucleotide polymorphisms (SNPs), DNA methylation and microRNA expression in different tissues (such as placenta, umbilical cord, adipose tissue, and peripheral blood), and microbial composition in the gut, oral cavity, and vagina from pregnant women with GDM, as well as the bacterial composition of the offspring. Altogether, these reports indicate that a number of SNPs are associated to GDM phenotypes and may predispose the development of the disease. However, extrinsic factors (lifestyle, nutrition) modulate, through epigenetic mechanisms, the risk of developing the disease, and some association exists between the microbial composition with GDM in an organ-specific manner. Genes, epigenetic signatures, and microbiota could be transferred to the offspring, increasing the possibility of developing chronic degenerative conditions through postnatal life.

(Modified from the IDF Diabetes Atlas, IDF 2021, with the permission of the IDF Atlas team)


Similar content being viewed by others
Data availability
No new data were created or analysed in this study. Data sharing is not applicable to this article.
References
International Diabetes Federation (2021) IDF Diabetes Atlas, tenth ed., Brussels, Belgium.
American Diabetes Association (2022) Classification and diagnosis of diabetes: standards of medical care in diabetes-2023. Diabetes Care 46(Suppl 1):S19–S40. https://doi.org/10.2337/dc23-S002
Catalano PM, Mcintyre HD, Cruickshank JK et al (2012) The hyperglycemia and adverse pregnancy outcome study. Diabetes Care 35:780–786. https://doi.org/10.2337/dc11-179
Lizárraga D, García-Gasca A (2021) The placenta as a target of epigenetic alterations in women with gestational diabetes mellitus and potential implications for the offspring. Epigenomes 5:13
Dias S, Pheiffer C, Abrahams Y, Rheeder P, Adam S (2018) Molecular biomarkers for gestational diabetes mellitus. Int J Mol Sci 19:2926. https://doi.org/10.3390/ijms19102926
Berkowitz GS, Lapinski RH, Wein R, Lee D (1992) Race/ethnicity and other risk factors for gestational diabetes. Am J Epidemiol 135:965–973. https://doi.org/10.1093/oxfordjournals.aje.a116408
Crusell MK, Hansen TH, Nielsen T et al (2018) Gestational diabetes is associated with change in the gut microbiota composition in third trimester of pregnancy and postpartum. Microbiome 6:89. https://doi.org/10.1186/s40168-018-0472-x
Franzago M, Fraticelli F, Stuppia L, Vitacolonna E (2019) Nutrigenetics, epigenetics and gestational diabetes: consequences in mother and child. Vitacolonnaa 14:215–235. https://doi.org/10.1080/15592294.2019.1582277
Gudsnuk K, Champagne FA (2012) Epigenetic influence of stress and the social environment. ILAR J 53:279–288. https://doi.org/10.1093/ilar.53.3-4.279
Tammen SA, Friso S, Choi SW (2013) Epigenetics: the link between nature and nurture. Mol Aspects Med 34:753–764. https://doi.org/10.1016/j.mam.2012.07.018
Mello CS, Carmo-Rodrigues MS, Filho HB, Melli LC, Tahan S, Pignatari AC, de Morais MB (2016) Gut microbiota differences in children from distinct socioeconomic levels living in the same urban area in Brazil. J Pediatr Gastroenterol Nutr 63:460–465. https://doi.org/10.1097/MPG.0000000000001186
Gomaa EZ (2020) Human gut microbiota/microbiome in health and diseases: a review. Antonie Van Leeuwenhoek 113:2019–2040. https://doi.org/10.1007/s10482-020-01474-7
Liang Y, Gong Y, Zhang X et al (2018) Dietary protein intake, meat consumption, and dairy consumption in the year preceding pregnancy and during pregnancy and their associations with the risk of gestational diabetes mellitus: a prospective cohort study in Southwest China. Front Endocrinol 9:596. https://doi.org/10.3389/fendo.2018.00596
Marí-Sanchis A, Díaz-Jurado G, Basterra-Gortari FJ, de la Fuente-Arrillaga C, Martínez-González MA, Bes-Rastrollo M (2017) Association between pre-pregnancy consumption of meat, iron intake, and the risk of gestational diabetes: the SUN project. Eur J Nutr 57:939–949. https://doi.org/10.1007/s00394-017-1377-3
Pinto RS, Minanni CA, de Araújo Lira AL, Passarelli M (2022) Advanced glycation end products: a sweet flavor that embitters cardiovascular disease. Int J Mol Sci 23:2404. https://doi.org/10.3390/ijms23052404
Sedaghat F, Akhoondan M, Ehteshami M, Aghamohammadi V, Ghanei N, Mirmiran P, Rashidkhani B (2017) Maternal dietary patterns and gestational diabetes risk: a case-control study. J Diabetes Res 2017:5173926. https://doi.org/10.1155/2017/5173926
Zhang C, Liu S, Solomon CG, Hu FB (2006) Dietary fiber intake, dietary glycemic load, and the risk for gestational diabetes mellitus. Diabetes Care 29(10):2223–2230
Mijatovic-Vukas J, Capling L, Cheng S et al (2018) Associations of diet and physical activity with risk for gestational diabetes mellitus: a systematic review and meta-analysis. Nutrients 10:698. https://doi.org/10.3390/nu10060698
Bar-Zeev Y, Haile ZT, Chertok IA (2020) Association between prenatal smoking and gestational diabetes mellitus. Obstet Gynecol 135:91–99. https://doi.org/10.1097/AOG.0000000000003602
Song Y, Wang L, Zheng D, Zeng L, Wang Y (2022) Sleep disturbances before pregnancy and subsequent risk of gestational diabetes mellitus. Nat Sci Sleep 14:1165–1174. https://doi.org/10.2147/NSS.S363792
Huvinena E, Grotenfelt NE, Eriksson JG et al (2016) Heterogeneity of maternal characteristics and impact on gestational diabetes (GDM) risk—Implications for universal GDM screening? Ann Med 48:52–58. https://doi.org/10.3109/07853890.2015.1131328
Devesa-Peiró A, Sánchez-Reyes JM, Díaz-Gimeno P (2020) Molecular biology approaches utilized in preimplantation genetics: real-time PCR, microarrays, next-generation sequencing, karyomapping, and others. In: García-Velasco JA, Seli E (eds) Human reproductive genetics. Academic Press, pp 49–67. https://doi.org/10.1016/C2018-0-00272-8
Hirschhorn JN, Daly MJ (2005) Genome-wide association studies for common diseases and complex traits. Nat Rev Genet 6:95–108. https://doi.org/10.1038/nrg1521
Grezzana GB, Da Costa JL, Portal VL (1992) Single-nucleotide polymorphisms: a perspective of cardiovascular prevention. Rev Assoc Med Bras 61:458–468. https://doi.org/10.1590/1806-9282.61.05.458
McCarthy MI (2010) Genomics, type 2 diabetes, and obesity. N Engl J Med 363:2339–2350. https://doi.org/10.1056/NEJMra0906948
Wu L, Cui L, Tam WH, Ma RC, Wang CC (2016) Genetic variants associated with gestational diabetes mellitus: a meta-analysis and subgroup analysis. Sci Rep 6:30539. https://doi.org/10.1038/srep30539
Akbaba G, Akbaba E, Sahin C, Kara M (2018) The relationship between gestational diabetes mellitus and selenoprotein-P plasma 1 (SEPP1) gene polymorphisms. Gynecol Endocrinol 34:849–852. https://doi.org/10.1080/09513590.2018.1460659
Beysel S, Eyerci N, Ulubay M et al (2019) Maternal genetic contribution to pre-pregnancy obesity, gestational weight gain, and gestational diabetes mellitus. Diabetol Metab Syndr 11:37. https://doi.org/10.1186/s13098-019-0434-x
Pawlik A, Teler J, Maciejewska A, Sawczuk M, Safranow K, Dziedziejko V (2017) Adiponectin and leptin gene polymorphisms in women with gestational diabetes mellitus. J Assist Reprod Genet 34:511–516. https://doi.org/10.1007/s10815-016-0866-2
Tarnowski M, Tkacz M, Dziedziejko V, Safranow K, Pawlik A (2017) COX2 and NOS3 gene polymorphisms in women with gestational diabetes. J Gene Med. https://doi.org/10.1002/jgm.2959
Tarnowski M, Malinowski D, Pawlak K, Dziedziejko V, Safranow K, Pawlik A (2017) GCK, GCKR, FADS1, DGKB/TMEM195 and CDKAL1 gene polymorphisms in women with gestational diabetes. Can J Diabetes 41:372–379. https://doi.org/10.1016/j.jcjd.2016.11.009
Tarnowski M, Malinowski D, Safranow K, Dziedziejko V, Pawlik A (2017) MTNR1A and MTNR1B gene polymorphisms in women with gestational diabetes. Gynecol Endocrinol 33:395–398. https://doi.org/10.1080/09513590.2016.1276556
Tarnowski M, Wieczorek A, Dziedziejko V et al (2017) IL16 and IL18 gene polymorphisms in women with gestational diabetes. Ginekol Pol 88:249–254. https://doi.org/10.5603/GP.a2017.0047
Teler J, Tarnowski M, Safranow K et al (2017) CCL2, CCL5, IL4 and IL15 gene polymorphisms in women with gestational diabetes mellitus. Horm Metab Res 49:10–15. https://doi.org/10.1055/s-0042-111436
Vejrazkova D, Lukasova P, Vankova M et al (2014) MTNR1B genetic variability is associated with gestational diabetes in Czech women. Int J Endocrinol 2014:508923. https://doi.org/10.1155/2014/508923
Bassols J, Megia A, Soriano-Rodríguez P et al (2013) A common gene variant in STK11 is associated with metabolic risk markers and diabetes during gestation. Fertil Steril 100:788–792. https://doi.org/10.1016/j.fertnstert.2013.04.037
Beltcheva O, Boyadzhieva M, Angelova O, Mitev V, Kaneva R, Atanasova I (2014) The rs266729 single-nucleotide polymorphism in the adiponectin gene shows association with gestational diabetes. Arch Gynecol Obstet 289:743–748. https://doi.org/10.1007/s00404-013-3029-z
Ding M, Chavarro J, Olsen S, Lin Y, Ley SH, Bao W, Rawal S, Grunnet LG, Thuesen AC, Mills JL et al (2018) Genetic variants of gestational diabetes mellitus: a study of 112 SNPs among 8,722 women in two independent populations. Diabetologia 61:1758–1768. https://doi.org/10.1007/s00125-018-4637-8
Franzago M, Fraticelli F, Marchetti D, Celentano C, Liberati M, Stuppia L, Vitacolonna E (2018) Nutrigenetic variants and cardio-metabolic risk in women with or without gestational diabetes. Diabetes Res Clin Pract 137:64–71. https://doi.org/10.1016/j.diabres.2018.01.001
Huopio H, Cederberg H, Vangipurapu J, Hakkarainen H, Paakkonen M, Kuulasmaa T, Heinonen S, Laakso M (2013) Association of risk variants for type 2 diabetes and hyperglycemia with gestational diabetes. Eur J Endocrinol 169:291–297. https://doi.org/10.1530/EJE-13-0286
Papadopoulou A, Lynch KF, Shaat N, Håkansson R, Ivarsson SA, Berntorp K, Agardh CD, Lernmark A (2011) Gestational diabetes mellitus is associated with TCF7L2 gene polymorphisms independent of HLA-DQB1*0602 genotypes and islet cell autoantibodies. Diabet Med 28:1018–1027. https://doi.org/10.1111/j.1464-5491.2011.03359.x
Popova PV, Klyushina AA, Vasilyeva LB et al (2017) Effect of gene-lifestyle interaction on gestational diabetes risk. Oncotarget 8:112024–112035
Fatima SS, Chaudhry B, Khan TA, Farooq S (2016) KCNQ1 rs2237895 polymorphism is associated with gestational diabetes in Pakistani women. Pak J Med Sci 32:1380–1385. https://doi.org/10.12669/pjms.326.11052
Hasanvand Z, Sadeghi A, Rezvanfar MR, Goodarzi MT, Rahmannezhad G, Mashayekhi FJ (2018) Association between chemerin rs17173608 and rs4721 gene polymorphisms and gestational diabetes mellitus in Iranian pregnant women. Gene 649:87–92. https://doi.org/10.1016/j.gene.2018.01.061
Takhshid MA, Haem Z, Aboualizadeh F (2015) The association of circulating adiponectin and + 45 T/G polymorphism of adiponectin gene with gestational diabetes mellitus in Iranian population. J Diabetes Metab Disord 14:30. https://doi.org/10.1186/s40200-015-0156-z
Aslani S, Hossein-nezhad A, Maghbooli Z, Mirzaei K, Karimi F (2011) Genetic variation in macrophage migration inhibitory factor associated with gestational diabetes mellitus and metabolic syndrome. Horm Metab Res 43:557–561. https://doi.org/10.1055/s-0031-1275706
Alharbi KK, Khan IA, Eldesouky MH, Al-Hakeem MM, Abotalib Z (2015) The genetic polymorphism in the STK11 does not affect gestational diabetes. Acta Biochim Pol 62:569–572. https://doi.org/10.18388/abp.2015_1025
Reyes-López R, Pérez-Luque E, Malacara JM (2014) Metabolic, hormonal characteristics and genetic variants of TCF7L2 associated with development of gestational diabetes mellitus in Mexican women. Diabetes Metab Res Rev 30:701–706. https://doi.org/10.1002/dmrr.2538
Anghebem-Oliveira MI, Webber S, Alberton D et al (2017) The GCKR gene polymorphism rs780094 is a risk factor for gestational diabetes in a Brazilian population. J Clin Lab Anal 31:e22035. https://doi.org/10.1002/jcla.22035
Zhang T, Zhao L, Wang S et al (2021) Common variants in NUS1 and GP2 Genes contributed to the risk of gestational diabetes mellitus. Front Endocrinol 12:685524. https://doi.org/10.3389/fendo.2021.685524
Jia Y, Shen Y, Shi X et al (2020) MTNR1B gene on susceptibility to gestational diabetes mellitus: a two-stage hospital-based study in Southern China. Mol Genet Genomics 295:1369–1378. https://doi.org/10.1007/s00438-020-01706-5
Li C, Qiao B, Qi W et al (2016) Association of macrophage migration inhibitory factor polymorphisms with gestational diabetes chinese. Gynecol Obstet Invest 81:84–89. https://doi.org/10.1159/000398796
Li C, Qiao B, Zhou Y, Qi W, Ma C, Zheng L (2020) Association of estrogen receptor α gene polymorphism and its expression with gestational diabetes mellitus. Gynecol Obstet Invest 85:26–33. https://doi.org/10.1159/000502378
Liu T, Deng JM, Liu YL, Chang L, Jiang YM (2020) The relationship between gestational diabetes mellitus and interleukin 1beta gene polymorphisms in southwest of China. Medicine 99:e22679. https://doi.org/10.1097/MD.0000000000022679
Wang X, Li W, Ma L et al (2015) Association study of the miRNA-binding site polymorphisms of CDKN2A/B genes with gestational diabetes mellitus susceptibility. Acta Diabetol 52:951–958. https://doi.org/10.1007/s00592-015-0768-2
Wang H, Yang W, Liu J, Leng J, Li W, Yu Z et al (2021) Serum concentrations of SFAs and CDKAL1 single-nucleotide polymorphism rs7747752 are related to an increased risk of gestational diabetes mellitus. Am J Clin Nutr 114(5):1698–1707. https://doi.org/10.1093/ajcn/nqab225
Zhang X, Sun L, Jin Z (2018) Effect of placental sex hormone-binding globulin single nucleotide polymorphism rs6259 on protein and function in gestational diabetes mellitus. Int J Mol Med 41:2927–2934. https://doi.org/10.3892/ijmm.2018.3503
Han Y, Zheng YL, Fan YP, Liu MH, Lu XY, Tao Q (2015) Association of adiponectin gene polymorphism 45TG with gestational diabetes mellitus diagnosed on the new IADPSG criteria, plasma adiponectin levels and adverse pregnancy outcomes. Clin Exp Med 15:47–53. https://doi.org/10.1007/s10238-014-0275-8
Lai S, Yan D, Xu J et al (2023) Genetic variants in epoxyeicosatrienoic acid processing and degradation pathways are associated with gestational diabetes mellitus. Nutr J 22(1):31. https://doi.org/10.1186/s12937-023-00862-9
Li C, Zhou Y, Qiao B, Xu L, Li Y, Li C (2019) Association between a melatonin receptor 1B genetic polymorphism and its protein expression in gestational diabetes mellitus. Reprod Sci 26:1382–1388. https://doi.org/10.1177/1933719118765983
Shi A, Wen J, Liu G et al (2016) Genetic variants in vitamin D signaling pathways and risk of gestational diabetes mellitus. Oncotarget 7:67788–67795. https://doi.org/10.18632/oncotarget.11984
Low CF, Tohit ER, Chong PP, Idris F (2011) Adiponectin SNP45TG is associated with gestational diabetes mellitus. Arch Gynecol Obstet 283:1255–1260. https://doi.org/10.1007/s00404-010-1548-4
Jamalpour S, Zain SM, Mosavat M, Mohamed Z, Omar SZ (2018) A case-control study and meta-analysis confirm glucokinase regulatory gene rs780094 is a risk factor for gestational diabetes mellitus. Gene 650:34–40. https://doi.org/10.1016/j.gene.2018.01.091
Shin HD, Park BL, Shin HJ et al (2010) Association of KCNQ1 polymorphisms with the gestational diabetes mellitus in Korean women. J Clin Endocrinol Metab 95:445–449. https://doi.org/10.1210/jc.2009-1393
Kim JY, Cheong HS, Park BL et al (2011) Melatonin receptor 1 B polymorphisms associated with the risk of gestational diabetes mellitus. BMC Med Genet 12:82. https://doi.org/10.1186/1471-2350-12-82
Kwak SH, Kim TH, Cho YM, Choi SH, Jang HC, Park KS (2010) Polymorphisms in KCNQ1 are associated with gestational diabetes in a korean population. Horm Res Paediatr 74:333–338. https://doi.org/10.1159/000313918
Kang J, Liu CH, Lee CN et al (2019) Novel interleukin-10 gene polymorphism is linked to gestational diabetes in Taiwanese Population. Front Genet 10:89. https://doi.org/10.3389/fgene.2019.00089
Amin US, Parvez N, Rahman TA, Hasan MR, Das KC, Jahan S et al (2022) CDKAL1 gene rs7756992 A/G and rs7754840 G/C polymorphisms are associated with gestational diabetes mellitus in a sample of Bangladeshi population: implication for future T2DM prophylaxis. Diabetol Metab Syndr 14:18. https://doi.org/10.1186/s13098-021-00782-w
de Melo SF, Frigeri HR, dos Santos-Weiss IC et al (2015) Polymorphisms in FTO and TCF7L2 genes of Euro-Brazilian women with gestational diabetes. Clin Biochem 48:1064–1067. https://doi.org/10.1016/j.clinbiochem.2015.06.013
Wang J, Kuusisto J, Vänttinen M et al (2007) Variants of transcription factor 7-like 2 (TCF7L2) gene predict conversion to type 2 diabetes in the finnish diabetes prevention study and are associated with impaired glucose regulation and impaired insulin secretion. Diabetologia 50(6):1192–1200. https://doi.org/10.1007/s00125-007-0656-6
Cropano C, Santoro N, Groop L et al (2017) The rs7903146 variant in the TCF7L2 gene increases the risk of prediabetes/Type 2 diabetes in obese adolescents by impairing β-cell function and hepatic insulin sensitivity. Diabetes Care 40(8):1082–1089. https://doi.org/10.2337/dc17-0290
Choi SW, Mak TS, O’Reilly PF (2020) Tutorial: a guide to performing polygenic risk score analyses. Nat Protoc 15(9):2759–2772. https://doi.org/10.1038/s41596-020-0353-1
Lamri A, Mao S, Desai D, Gupta M, Paré G, Anand SS (2020) Fine-tuning of genome-wide polygenic risk scores and prediction of gestational diabetes in South Asian Women. Sci Rep 10(1):8941. https://doi.org/10.1038/s41598-020-65360-y
Hodgson S, Huang QQ, Sallah N et al (2022) Integrating polygenic risk scores in the prediction of type 2 diabetes risk and subtypes in British Pakistanis and Bangladeshis: a population-based cohort study. PLoS Med 19(5):e1003981. https://doi.org/10.1371/journal.pmed.1003981
National Health Service UK (2019) Gestational Diabetes National Health Service. https://www.nhs.uk/conditions/gestational-diabetes/treatment/. Accessed 06 Aug 2022
Young BC, Ecker JL (2013) Fetal macrosomia and shoulder dystocia in women with gestational diabetes: risks amenable to treatment? Curr Diabetes Rep 13:12–18. https://doi.org/10.1007/s11892-012-0338-8
Aye IL, Powell TL, Jansson T (2013) Review: adiponectin—The missing link between maternal adiposity, placental transport and fetal growth? Placenta 34:S40–S45. https://doi.org/10.1016/j.placenta.2012.11.024
Beysel S, Pinarli FA, Eyerci N et al (2020) HNF1A gene p.I27L is associated with co-existing preeclampsia in gestational diabetes mellitus. Gynecol Endocrinol 36(6):530–534. https://doi.org/10.1080/09513590.2019.1698023
Bellamy L, Casas JP, Hingorani AD, Williams D (2009) Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet 373:1773–1779. https://doi.org/10.1016/S0140-6736(09)60731-5
Chen Y, Liu H, Wang L et al (2019) Lifestyle intervention modifies the effect of the MC4R genotype on changes in insulin resistance among women with prior gestational diabetes: Tianjin gestational diabetes mellitus prevention program. Am J Clin Nutr 11:750–758. https://doi.org/10.1093/ajcn/nqz121
Liang Z, Wang L, Liu H et al (2020) Genetic susceptibility, lifestyle intervention and glycemic changes among women with prior gestational diabetes. Clin Nutr 39:2144–2150. https://doi.org/10.1016/j.clnu.2019.08.032
Barker DJ (2004) The developmental origins of adult disease. J Am Coll Nutr 23:588S-595S. https://doi.org/10.1080/07315724.2004.10719428
Ryznar RJ, Phibbs L, Van Winkle LJ (2021) Epigenetic Modifications at the Center of the Barker Hypothesis and Their Transgenerational Implications. Int J Environ Res Public Health 18:12728. https://doi.org/10.3390/ijerph182312728
Gaccioli F, Lager S, Powell TL, Jansson T (2012) Placental transport in response to altered maternal nutrition. J Dev Orig Health Dis 4:1–15. https://doi.org/10.1017/S2040174412000529
Lager S, Powell TL (2012) Regulation of nutrient transport across the placenta. J Pregnancy 2012:1–14. https://doi.org/10.1155/2012/179827
Gallo LA, Barrett HL, Dekker Nitert M (2017) Review: Placental transport and metabolism of energy substrates in maternal obesity and diabetes. Placenta 54:59–67. https://doi.org/10.1016/j.placenta.2016.12.006
Feinberg AP (2007) Phenotypic plasticity and the epigenetics phenotypic plasticity and the epigenetics. Nature 447:433–440. https://doi.org/10.1038/nature05919
Jang HS, Shin WJ, Lee JE, Do JT (2017) CpG and Non-CpG methylation in epigenetic gene regulation and brain function. Genes 8:1–20. https://doi.org/10.3390/genes8060148
Li Z, Zhao P, Xia Q (2019) Epigenetic methylations on N6-adenine and N6-adenosine with the same input but different output. Int J Mol Sci 20:1–13. https://doi.org/10.3390/ijms20122931
Rosik J, Szostak B, Machaj F, Pawlik A (2020) The role of genetics and epigenetics in the pathogenesis of gestational diabetes mellitus. Ann Hum Genet 84:114–124. https://doi.org/10.1111/ahg.12356
Lesseur C, Armstrong DA, Paquette AG, Li Z, Padbury JF, Marsit CJ (2014) Maternal obesity and gestational diabetes are associated with placental leptin DNA methylation. Am J Obstet Gynecol 211:654.e1-654.e9. https://doi.org/10.1016/j.ajog.2014.06.037
Houde AA, St-Pierre J, Hivert MF et al (2014) Placental lipoprotein lipase DNA methylation levels are associated with gestational diabetes mellitus and maternal and cord blood lipid profiles. J Dev Orig Health Dis 5:132–141. https://doi.org/10.1017/S2040174414000038
Gagné-Ouellet V, Houde AA, Guay SP et al (2017) Placental lipoprotein lipase DNA methylation alterations are associated with gestational diabetes and body composition at 5 years of age. Epigenetics 12:616–625. https://doi.org/10.1080/15592294.2017.1322254
El Hajj N, Pliushch G, Schneider E et al (2012) Metabolic programming of MEST DNA methylation by intrauterine exposure to gestational diabetes mellitus. Diabetes 62:1320–1328. https://doi.org/10.2337/db12-0289
Nomura Y, Lambertini L, Rialdi A et al (2013) Global methylation in the placenta and umbilical cord blood from pregnancies with maternal gestational diabetes, preeclampsia, and obesity. Reprod Sci 21:131–137. https://doi.org/10.1177/1933719113492206
Reichetzeder C, Putra SE, Pfab T et al (2016) Increased global placental DNA methylation levels are associated with gestational diabetes. Clin Epigenetics 8:82. https://doi.org/10.1186/s13148-016-0247-9
Finer S, Mathews C, Lowe R et al (2015) Maternal gestational diabetes is associated with genome-wide DNA methylation variation in placenta and cord blood of exposed offspring. Hum Mol Genet 24:3021–3029. https://doi.org/10.1093/hmg/ddv013
Haertle L, El Hajj N, Dittrich M et al (2017) Epigenetic signatures of gestational diabetes mellitus on cord blood methylation. Clin Epigenetics 9:28. https://doi.org/10.1186/s13148-017-0329-3
Hivert MF, Cardenas A, Allard C et al (2020) Interplay of placental DNA methylation and maternal insulin sensitivity in pregnancy. Diabetes 69:484–492. https://doi.org/10.2337/db19-0798
Howe CG, Cox B, Fore R et al (2020) Maternal gestational diabetes mellitus and newborn DNA methylation: findings from the pregnancy and childhood epigenetics consortium. Diabetes Care 43:98–105. https://doi.org/10.2337/dc19-0524
Kim E, Kwak SH, Chung HR et al (2017) DNA methylation profiles in sibling pairs discordant for intrauterine exposure to maternal gestational diabetes. Epigenetics 12:825–832. https://doi.org/10.1080/15592294.2017.1370172
Dias S, Adam S, Abrahams Y, Rheeder P, Pheiffer C (2021) Adiponectin DNA methylation in South African women with gestational diabetes mellitus: effects of HIV infection. PLoS ONE 16:e0248694. https://doi.org/10.1371/journal.pone.0248694
Kang J, Lee CN, Li HY, Hsu KH, Lin SY (2017) Genome-wide DNA methylation variation in maternal and cord blood of gestational diabetes population. Diabetes Res Clin Pract 132:127–136. https://doi.org/10.1016/j.diabres.2017.07.034
Wang X, Huang J, Zheng Y et al (2021) Study on the relationship between DNA methylation of target CpG sites in peripheral blood and gestational diabetes during early pregnancy. Sci Rep 11:20455. https://doi.org/10.1038/s41598-021-99836-2
Dias S, Adam S, Van Wyk N, Rheeder P, Louw J, Pheiffer C (2019) Global DNA methylation profiling in peripheral blood cells of South African women with gestational diabetes mellitus. Biomarkers 24:225–231. https://doi.org/10.1080/1354750X.2018.1539770
Kang J, Lee CN, Li HY, Hsu KH, Wang SH, Lin SY (2018) Association of interleukin-10 methylation levels with gestational diabetes in a taiwanese population. Front Genet 9:222. https://doi.org/10.3389/fgene.2018.00222
Wu P, Farrell WE, Haworth KE et al (2018) Maternal genome-wide DNA methylation profiling in gestational diabetes shows distinctive disease-associated changes relative to matched healthy pregnancies. Epigenetics 13:122–128. https://doi.org/10.1080/15592294.2016.1166321
Ott R, Stupin JH, Melchior K et al (2018) Alterations of adiponectin gene expression and DNA methylation in adipose tissues and blood cells are associated with gestational diabetes and neonatal outcome. Clin Epigenetics 10:131. https://doi.org/10.1186/s13148-018-0567-z
Rancourt RC, Ott R, Schellong K et al (2021) Altered SOCS3 DNA methylation within exon 2 is associated with increased mRNA expression in visceral adipose tissue in gestational diabetes. Epigenetics 16:488–494. https://doi.org/10.1080/15592294.2020.1805695
Shi W, Lefebvre L, Yu Y et al (2004) Loss-of-imprinting ofPeg1 in mouse interspecies hybrids is correlated with altered growth. Genestics 39:65–72. https://doi.org/10.1002/gene.20027
Chinetti G, Staels JC (2000) Peroxisome proliferator-activated receptors (PPARs): nuclear receptors at the crossroads between lipid metabolism and inflammation. Inflamm Res 49:497–505. https://doi.org/10.1007/s000110050622
Pérez-Pérez A, Toro A, Vilariño-García T et al (2017) Leptin action in normal and pathological pregnancies. J Cell Mol Med 22:716–727. https://doi.org/10.1111/jcmm.13369
Awamleh Z, Butcher DT, Hanley A, Retnakaran R, Haertle L, Haaf T (2021) Exposure to gestational diabetes mellitus (GDM) alters DNA methylation in placenta and fetal cord blood. Diabetes Res Clin Pract 174:108690. https://doi.org/10.1016/j.diabres.2021.108690
Franzago M, Fraticelli F, Marchioni M et al (2021) Fat mass and obesity-associated (FTO) gene epigenetic modifications in gestational diabetes: new insights and possible pathophysiological connections. Acta Diabetol 58(8):997–1007. https://doi.org/10.1007/s00592-020-01668-5
Franzago M, Porreca A, D’Ardes M et al (2022) The obesogenic environment: epigenetic modifications in placental melanocortin 4 receptor gene connected to gestational diabetes and smoking. Front Nutr 9:879526. https://doi.org/10.3389/fnut.2022.879526
Hjort L, Novakovic B, Cvitic S, Saffery R, Damm P, Desoye G (2022) Placental DNA methylation in pregnancies complicated by maternal diabetes and/or obesity: state of the art and research gaps. Epigenetics 17(13):2188–2208. https://doi.org/10.1080/15592294.2022.2111755
Hjort L, Martino D, Grunnet LG et al (2018) Gestational diabetes and maternal obesity are associated with epigenome-wide methylation changes in children. JCI Insight 3:122572. https://doi.org/10.1172/jci.insight.122572
Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cel 116:281–297. https://doi.org/10.1016/s0092-8674(04)00045-5
Légaré C, Clément AA, Desgagné V et al (2022) Human plasma pregnancy-associated miRNAs and their temporal variation within the first trimester of pregnancy. Reprod Biol Endocrinol 20:14. https://doi.org/10.1186/s12958-021-00883-1
Nelissen EC, van Montfoort AP, Dumoulin JC, Evers JL (2011) Epigenetics and the placenta. Hum Reprod Update 17:397–417. https://doi.org/10.1093/humupd/dmq052
Liang J, Wang S, Wang Z (2017) Role of microRNAs in embryo implantation. Reprod Biol Endocrinol 15:90. https://doi.org/10.1186/s12958-017-0309-7
Cook J, Bennett PR, Kim SH et al (2019) First trimester circulating MicroRNA biomarkers predictive of subsequent preterm delivery and cervical shortening. Sci Rep 9:5861. https://doi.org/10.1038/s41598-019-42166-1
Fu G, Brkić J, Hayder H, Peng C (2013) MicroRNAs in human placental development and pregnancy complications. Int J Mol Sci 14:5519–5544. https://doi.org/10.3390/ijms14035519
Filardi T, Catanzaro G, Mardente S et al (2020) Non-coding RNA: role in gestational diabetes pathophysiology and complications. Int J Mol Sci 21:4020. https://doi.org/10.3390/ijms21114020
Zhao C, Dong J, Jiang T et al (2011) Early second-trimester serum mirna profiling predicts gestational diabetes mellitus. PLoS ONE 6:e23925. https://doi.org/10.1371/journal.pone.0023925
Zhao C, Zhang T, Shi Z, Ding H, Ling X (2014) MicroRNA-518d regulates PPARα protein expression in the placentas of females with gestational diabetes mellitus. Mol Med Rep 9:2085–2090. https://doi.org/10.3892/mmr.2014.2058
Floris I, Descamps B, Vardeu A et al (2015) Gestational diabetes mellitus impairs fetal endothelial cell functions through a mechanism involving microRNA-101 and histone methyltransferase enhancer of zester homolog-2. Arterioscler Thromb Vasc Biol 35:664–674. https://doi.org/10.1161/ATVBAHA.114.304730
Li J, Song L, Zhou L et al (2015) A MicroRNA signature in gestational diabetes mellitus associated with risk of macrosomia. Cell Physiol Biochem 37:243–252. https://doi.org/10.1159/000430349
Muralimanoharan S, Maloyan A, Myatt L (2016) Mitochondrial function and glucose metabolism in the placenta with gestational diabetes mellitus: role of miR-143. Clin Sci (Lond) 130:931–941. https://doi.org/10.1042/CS20160076
Tryggestad JB, Vishwanath A, Jiang S et al (2016) Influence of gestational diabetes mellitus on human umbilical vein endothelial cell miRNA. Clin Sci (Lond) 130:1955–1967. https://doi.org/10.1042/CS20160305
Cao JL, Zhang L, Li J et al (2016) Up-regulation of miR-98 and unraveling regulatory mechanisms in gestational diabetes mellitus. Sci Rep 6:32268. https://doi.org/10.1038/srep32268
Sebastiani G, Guarino E, Grieco GE, Formichi C, Poggi CD, Ceccarelli E (2017) Circulating microRNA (miRNA) expression profiling in plasma of patients with gestational diabetes mellitus reveals upregulation of miRNA miR-330-3p. Front Endocrinol (Lausanne) 8:345. https://doi.org/10.3389/fendo.2017.00345
He Y, Bai J, Liu P et al (2017) miR-494 protects pancreatic β-cell function by targeting PTEN in gestational diabetes mellitus. Excli J 16:1297–1307. https://doi.org/10.17179/excli2017-491
Ding R, Guo F, Zhang Y et al (2018) Integrated transcriptome sequencing analysis reveals role of miR-138-5p/TBL1X in placenta from gestational diabetes mellitus. Cell Physiol Biochem 51:630–646. https://doi.org/10.1159/000495319
Li L, Wang S, Li H et al (2018) microRNA-96 protects pancreatic β-cell function by targeting PAK1 in gestational diabetes mellitus. BioFactors 44:539–547. https://doi.org/10.1002/biof.1461
Nair S, Jayabalan N, Guanzon D et al (2018) Human placental exosomes in gestational diabetes mellitus carry a specific set of miRNAs associated with skeletal muscle insulin sensitivity. Clin Sci (Lond) 132:2451–2467. https://doi.org/10.1042/CS20180487
Peng HY, Li HP, Li MQ (2018) High glucose induces dysfunction of human umbilical vein endothelial cells by upregulating miR-137 in gestational diabetes mellitus. Microvasc Res 118:90–100. https://doi.org/10.1016/j.mvr.2018.03.002
Stirm L, Huypens P, Sass S et al (2018) Maternal whole blood cell miRNA-340 is elevated in gestational diabetes and inversely regulated by glucose and insulin. Sci Rep 8:1366. https://doi.org/10.1038/s41598-018-19200-9
Wang P, Wang Z, Liu G et al (2019) miR-657 promotes macrophage polarization toward M1 by targeting FAM46C in gestational diabetes mellitus. Mediators Inflamm 13:4851214. https://doi.org/10.1155/2019/4851214
Zhou X, Xiang C, Zheng X (2019) miR-132 serves as a diagnostic biomarker in gestational diabetes mellitus and its regulatory effect on trophoblast cell viability. Diagn Pathol 14:119. https://doi.org/10.1186/s13000-019-0899-9
Feng Y, Qu X, Chen Y et al (2020) MicroRNA-33a-5p sponges to inhibit pancreatic β-cell function in gestational diabetes mellitus LncRNA DANCR. Reprod Biol Endocrinol 18:61. https://doi.org/10.1186/s12958-020-00618-8
Guan CY, Tian S, Cao JL, Wang XQ, Ma X, Xia HF (2020) Down-regulated miR-21 in gestational diabetes mellitus placenta induces PPAR-α to inhibit cell proliferation and infiltration. Diabetes Metab Syndr Obes 13:3009–3034. https://doi.org/10.2147/DMSO.S253920
Sun DG, Tian S, Zhang L et al (2020) The miRNA-29b is downregulated in placenta during gestational diabetes mellitus and may alter placenta development by regulating trophoblast migration and invasion through a HIF3A-dependent mechanism. Front Endocrinol (Lausanne) 11:169. https://doi.org/10.3389/fendo.2020.00169
Tang L, Li P, Li L (2020) Whole transcriptome expression profiles in placenta samples from women with gestational diabetes mellitus. J Diabetes Investig 11:1307–1317. https://doi.org/10.1111/jdi.13250
Sørensen AE, van Poppel MN et al (2021) The predictive value of miR-16, -29a and -134 for early identification of gestational diabetes: A nested analysis of the DALI cohort. Cells 10:170. https://doi.org/10.3390/cells10010170
Zhang L, Zhang T, Sun D et al (2021) Diagnostic value of dysregulated miRNAs in the placenta and circulating exosomes in gestational diabetes mellitus. J Diabetes Investig 12:1490–1500. https://doi.org/10.1111/jdi.13493
Huang X, Liu G, Guo J, Su Z (2018) The PI3K/AKT pathway in obesity and type 2 diabetes. Int J Biol Sci 14:1483–1496. https://doi.org/10.7150/ijbs.27173
DeWaal D, Nogueira V, Terry AR et al (2018) Hexokinase-2 depletion inhibits glycolysis and induces oxidative phosphorylation in hepatocellular carcinoma and sensitizes to metformin. Nat Commun 9:446. https://doi.org/10.1038/s41467-017-02733-4
Burrows TD, King A, Loke YW (1996) Trophoblast migration during human placental implantation. Hum Reprod Update 2:307–321. https://doi.org/10.1093/humupd/2.4.307
Hunter RW, Treebak JT, Wojtaszewski JF, Sakamoto K (2011) Molecular mechanism by which AMP-activated protein kinase activation promotes glycogen accumulation in muscle. Diabetes 60:766–774. https://doi.org/10.2337/db10-1148
Shirwany NA, Zou MH (2014) AMPK: A cellular metabolic and redox sensor. A minireview. Front Biosci (Landmark Ed) 19:447–474. https://doi.org/10.2741/4218
Maranduba CM, De Castro SB, de Souza GT et al (2015) Intestinal microbiota as modulators of the immune system and neuroimmune system: impact on the host health and homeostasis. J Immunol Res 2015:931574. https://doi.org/10.1155/2015/931574
Wang J, Zheng J, Shi W et al (2018) Dysbiosis of maternal and neonatal microbiota associated with gestational diabetes mellitus. Gut 67:1614–1625. https://doi.org/10.1136/gutjnl-2018-315988
Crusell MK, Brink LR, Nielsen T et al (2020) Gestational diabetes and the human salivary microbiota: a longitudinal study during pregnancy and postpartum. BMC Pregnancy Childbirth 20:69. https://doi.org/10.1186/s12884-020-2764-y
Huang X, Huang X, Huang Y et al (2023) The oral microbiome in autoimmune diseases: Friend or foe? J Transl Med 21(1):211. https://doi.org/10.1186/s12967-023-03995-x
Chen C, Hemme C, Beleno J et al (2018) Oral microbiota of periodontal health and disease and their changes after nonsurgical periodontal therapy. ISME J 12(5):1210–1224. https://doi.org/10.1038/s41396-017-0037-1
Chattopadhyay I, Verma M, Panda M (2019) Role of oral microbiome signatures in diagnosis and prognosis of oral cancer. Technol Cancer Res Treat 18:1533033819867354. https://doi.org/10.1177/1533033819867354
Cortez RV, Taddei CR, Sparvoli LG et al (2019) Microbiome and its relation to gestational diabetes. Endocrine 64:254–264. https://doi.org/10.1007/s12020-018-1813-z
Zhang X, Liao Q, Wang F, Li D (2018) Association of gestational diabetes mellitus and abnormal vaginal flora with adverse pregnancy outcomes. Medicine 97:e11891. https://doi.org/10.1097/MD.0000000000011891
Aleshkin VA, Voropaeva EA, Shenderov BA (2006) Vaginal microbiota in healthy women and patients with bacterial vaginosis and nonspecific vaginitis. Microb Ecol Health Dis 18(2):71–74. https://doi.org/10.1080/17482960600891473
Razzak MS, Al-Charrakh AH, Al-Greitty BH (2011) Relationship between lactobacilli and opportunistic bacterial pathogens associated with vaginitis. N Am J Med Sci 3(4):185–192. https://doi.org/10.4297/najms.2011.3185
Kalia N, Singh J, Kaur M (2020) Microbiota in vaginal health and pathogenesis of recurrent vulvovaginal infections: a critical review. Ann Clin Microbiol Antimicrob 19(1):5. https://doi.org/10.1186/s12941-020-0347-4
Roachford OSE, Alleyne AT, Nelson KE (2022) Insights into the vaginal microbiome in a diverse group of women of African, Asian and European ancestries. PeerJ 10:e14449. https://doi.org/10.7717/peerj.14449
Rafat D, Singh S, Nawab T, Khan F, Khan AU, Khalid S (2021) Association of vaginal dysbiosis and gestational diabetes mellitus with adverse perinatal outcomes. Int J Gynaecol Obstet 8:70–78. https://doi.org/10.1002/ijgo.13945
Plows JF, Stanley JL, Baker PN, Reynolds CM, Vickers MH (2018) The pathophysiology of gestational diabetes mellitus. Int J Mol Sci 19:3342. https://doi.org/10.3390/ijms19113342
Krajmalnik-Brown R, Iihan KE, Kang DW, DiBaise JK (2012) Effects of gut microbes on nutrient absorption and energy regulation. Nutr Clin Pract 27:201–214. https://doi.org/10.1177/0884533611436116
Kuang YS, Lu JH, Li SH et al (2017) Connections between the human gut microbiome and gestational diabetes mellitus. Gigascience 6:1–12. https://doi.org/10.1093/gigascience/gix058
Su Y, Wang HK, Gan XP et al (2021) Alterations of gut microbiota in gestational diabetes patients during the second trimester of pregnancy in the Shanghai Han population. J Transl Med 19:366. https://doi.org/10.1186/s12967-021-03040-9
Xu Y, Zhang M, Zhang J et al (2020) Differential intestinal and oral microbiota features associated with gestational diabetes and maternal inflammation. Am J Physiol Endocrinol Metab 319:E247–E253. https://doi.org/10.1152/ajpendo.00266.2019
Gomez-Arango LF, Barrett HL, Wilkinson SA et al (2018) Low dietary fiber intake increases Collinsella abundance in the gut microbiota of overweight and obese pregnant women. Gut Microbes 9(3):189–201. https://doi.org/10.1080/19490976.2017.1406584
Swidsinski A, Weber J, Loening-Baucke V, Hale LP, Lochs H (2005) Spatial organization and composition of the mucosal flora in patients with inflammatory bowel disease. J Clin Microbiol 43(7):3380–3389. https://doi.org/10.1128/JCM.43.7.3380-3389.2005
Mokkala K, Houttu N, Vahlberg T, Munukka E, Rönnemaa T, Laitinen K (2017) Gut microbiota aberrations precede diagnosis of gestational diabetes mellitus. Acta Diabetol 54:1147–1149. https://doi.org/10.1007/s00592-017-1056-0
Gomez-Arango LF, Barrett HL, McIntyre HD, Callaway LK, Morrison M, Nitert MD (2016) Connections between the gut microbiome and metabolic hormones in early pregnancy in overweight and obese women. Diabetes 65:2214–2223. https://doi.org/10.2337/db16-0278
Liu H, Pan LL, Lv S et al (2019) Alterations of gut microbiota and blood lipidome in gestational diabetes mellitus with hyperlipidemia. Front Physiol 10:1015. https://doi.org/10.3389/fphys.2019.01015
Fugmann M, Breier M, Rottenkolber M et al (2015) The stool microbiota of insulin resistant women with recent gestational diabetes, a high risk group for type 2 diabetes. Sci Rep 5:13212. https://doi.org/10.1038/srep13212
Hasan S, Aho V, Pereira P et al (2018) Gut microbiome in gestational diabetes: a cross-sectional study of mothers and offspring 5 years postpartum. Acta Obstet Gynecol Scand 97:38–46. https://doi.org/10.1111/aogs.13252
Stinson LF, Payne MS, Keelan JA (2017) Planting the seed: origins, composition, and postnatal health significance of the fetal gastrointestinal microbiota. Crit Rev Microbiol 43:352–369. https://doi.org/10.1080/1040841X.2016.1211088
Soderborg TK, Carpenter CM, Ir D et al (2020) Gestational diabetes is uniquely associated with altered early seeding of the infant gut microbiota. Front Endocrinol 11:603021. https://doi.org/10.3389/fendo.2020.603021
Acknowledgements
The authors would like to thank the National Council for Science and Technology (CONACYT) for the doctoral scholarship granted to Dennise Lizárraga.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
DL and AGG contributed to preparation, edition, and revision of the manuscript. BGG, TGG, AAS, and LC contributed to deep revision of the manuscript, according to their main areas of expertise. ASO added a clinical perspective to the review.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
Not applicable.
Informed consent
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article belongs to the topical collection Pregnancy and Diabetes, managed by Antonio Secchi and Marina Scavini
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lizárraga, D., Gómez-Gil, B., García-Gasca, T. et al. Gestational diabetes mellitus: genetic factors, epigenetic alterations, and microbial composition. Acta Diabetol 61, 1–17 (2024). https://doi.org/10.1007/s00592-023-02176-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-023-02176-y