Abstract
Backgrounds
Two SNPs in melatonin receptor 1B gene, rs10830963 and rs1387153 showed significant associations with fasting plasma glucose levels and the risk of Type 2 Diabetes Mellitus (T2DM) in previous studies. Since T2DM and gestational diabetes mellitus (GDM) share similar characteristics, we suspected that the two genetic polymorphisms in MTNR1B may be associated with GDM, and conducted association studies between the polymorphisms and the disease. Furthermore, we also examined genetic effects of the two polymorphisms with various diabetes-related phenotypes.
Methods
A total of 1,918 subjects (928 GDM patients and 990 controls) were used for the study. Two MTNR1B polymorphisms were genotyped using TaqMan assay. The allele distributions of SNPs were evaluated by x 2 models calculating odds ratios (ORs), 95% confidence intervals (CIs), and corresponding P values. Multiple regressions were used for association analyses of GDM-related traits. Finally, conditional analyses were also performed.
Results
We found significant associations between the two genetic variants and GDM, rs10830963, with a corrected P value of 0.0001, and rs1387153, with the corrected P value of 0.0008. In addition, we also found that the two SNPs were associated with various phenotypes such as homeostasis model assessment of beta-cell function and fasting glucose levels. Further conditional analyses results suggested that rs10830963 might be more likely functional in case/control analysis, although not clear in GDM-related phenotype analyses.
Conclusion
There have been studies that found associations between genetic variants of other genes and GDM, this is the first study that found significant associations between SNPs of MTNR1B and GDM. The genetic effects of two SNPs identified in this study would be helpful in understanding the insight of GDM and other diabetes-related disorders.
Similar content being viewed by others
Background
The prevalence of type 2 diabetes mellitus (T2DM) in Korean population has dramatically increased over last decades. Although Asian populations traditionally had a low percentage of T2DM patients, it has increased drastically in recent decades. This is largely due to the fact that Asian countries are adopting western lifestyle and diets. However, a recent discovery of diabetes-susceptible loci on human chromosomes suggest that genetic factors may also play a role in the disease development [1].
Gestational diabetes mellitus (GDM) is a condition in which pregnant women exhibit glucose intolerance in various degrees [2], affecting approximately 2-14% of pregnancies [1, 3, 4]. Women with GDM show similar physiological and genetic characteristics found in diabetes outside of pregnancy, and not surprisingly, women with GDM possess higher risk for developing T2DM when they are not pregnant. Therefore, studying GDM is a good way to study early pathogenesis of diabetes and possibly develop treatment or remedy for the disease [5]. However, while genetic studies on T2DM are very robust [6, 7], there are relatively fewer genetic studies for GDM.
Advance of technology in the genetics field has provided us with a number of useful tools to study human genome. Among them, genome wide association studies (GWAS) are a powerful and useful way to detect genes associated with various diseases, including diabetes. Recently, a couple of studies have revealed that the genetic variants in melatonin receptor 1 B (MTNR1B) gene are associated with T2DM and fasting glucose levels [8, 9]. MTNR1B gene encodes MT2 protein which, along with MT1 protein encoded by MTNR1A, is one of two high-affinity forms of melatonin receptor. This gene product is also an integral membrane protein forming a G-protein coupled 7-transmembrane receptor. Melatonin, also known chemically as N-acetyl-5-methoxytryptamine, is a primary neurohormone secreted by pineal gland. It is mostly found in retina and brain, and its main function is thought to be the regulation of circadian rhythm by translating photoperiodic information from the eyes to the brain. There have been some studies suggesting that insulin level is regulated by circadian clock. Furthermore, T2DM patients have exhibited impaired melatonin secretion and circadian rhythm [10].
To date, several studies have shown the association between MTNR1B and T2DM [6, 11], but there has yet to be a study which looked into the association between MTNR1B and GDM. Since GDM shares many clinical features with T2DM, there is a high possibility that MTNR1B is associated with predisposition of GDM. Therefore, we conducted an association study between two polymorphisms of MTNR1B, which were previously associated with T2DM, using 928 GDM patients and 990 controls. In addition, we also examined the possible association between the two SNPs of MTNR1B and clinical phenotypes related to GDM, such as insulin sensitivity and beta-cell function.
Methods
Subjects
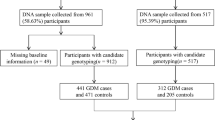
All 1,918 subjects included in this study were of Korean ethnic origin and recruited from Cheil General Hospital in Seoul, Korea, from 2003 to 2009. The clinical profiles of the patients are summarized in Table 1. All pregnant women who had not been previously diagnosed with T2DM were screened for GDM using a universal two-step GDM screening program at 24-28 week during gestation (GDM patients mean gestational week = 26.03 ± 2.69, NGT (Normal glucose tolerance) patients mean gestational week = 26.12 ± 1.69, Table 1). The first step was a 50-g glucose challenge test; if the result was positive (plasma glucose levels over 7.8 mmol/liter) after 1 hour, it was followed by conducting a 100-g, 3-hour oral glucose tolerance test (OGTT) after overnight fast according to criteria outlined by Carpenter and Coustan [12]. The threshold glucose values were as follows: fasting, at least 5.3 mmol/liter, 1 hour, at least 10.0 mmol/liter, 2 hours, at least 8.6 mmol/liter, and 3 hours, at least 7.8 mmol/liter. Plasma glucose concentrations were measured by the glucose oxidase method using a YSI 2300 STAT (YSI; Yellow Springs, Ohio). Insulin concentrations were measured using a human-specific radioimmunoassay kit (Linco Research, St. Charles, MO). If two or more of the glucose values were met or exceeded the above thresholds, GDM was diagnosed. A total of 928 GDM women were included in this study. 299 who were negative for 50-gr glucose challenge test and 691 who were pregnant and identified as normal glucose tolerant after undergoing the 100 gr. Glucose challenge test. The homeostasis model assessment (HOMA) index was used to calculate beta-cell function (HOMA-B) and insulin resistance (HOMA-IR) in various populations [13, 14]. Our study conforms to the Helsinki declaration and also to the Korean legislation. The institutional review board of Cheil General Hospital approved the study, and all subjects in the study provided informed consent.
SNP genotyping
Two polymorphisms of MTNR1B previously reported in a T2DM association study were selected and were genotyped using a TaqMan [15] assay in the Korean population. Genotyping quality control was performed in 10% of the samples by duplicate checking (rate of concordance in duplicates > 99%). The genotyping call success rates were 98.07% and 97.71% for rs1387153 and rs10830963, respectively. The probes used were C_1932612_10 for rs1387153 and C_3256858_10 for rs10830963.
Statistical analyses
The allele distributions of polymorphisms among GDM patients and normal subjects were evaluated by x 2 models calculating odds ratios (ORs), 95% confidence intervals (CIs), and corresponding P values. We used SAS version 9.1 (SAS Inc., Cary, NC) for the calculation. Multiple regressions were used for association analyses of GDM-related phenotypes adjusting for age and body mass index (BMI) as covariates, also using SAS version 9.1. Linkage disequilibrium between the two SNPs were calculated by the Haploview v4.1 software downloaded from the Broad Institute http://www.broadinstitute.org/mpg/haploview[16]. Statistical power of association was calculated by using Power for Genetic Association software [17]. For the calculation, disease prevalence of GDM was estimated to be 3%, based on previous researches [18], with risk allele frequencies of 0.503 and 0.521 for rs1387153 and rs10830963 respectively, and odd ratios of 1.3 and 1.35, also for respective polymorphisms. With these parameters, it was calculated that our sample of 928 cases and 990 controls would have over 90% statistical power with a type I error rate of 0.05. In order to correct the data for multiple testing, Bonferroni correction was applied. Also, we used PHASE software for haplotype inference [19], and inferred haplotypes were analyzed using SAS version 9.1 for the logistic analyses.
Also, we used PHASE software to estimate individual haplotypes and their frequencies, which uses a Bayesian approach. Individuals with phase probabilities less than 97% were excluded in analysis. To analyze the associations of haplotypes, we used Haplo.stats http://mayoresearch.mayo.edu/schaid_lab/software.cfm, which provides several haplotype-specific tests for association, as well as adjustment for non-genetic covariates and computation of simulation P-values. We also conducted conditional analyses with PLINK software http://pngu.mgh.harvard.edu/~purcell/plink/.
Results
Nine hundred and twenty eight GDM patients were recruited for the present study, and we also recruited 990 pregnant women with normal glucose tolerance as controls. The clinical profiles of the subjects are summarized in Table 1, with characteristics related with T2DM such as the area under glucose curve (AUC-G), fasting plasma insulin (FPI), and fasting plasma glucose (FPG). We also obtained homeostatic model assessment data for both groups in beta-cell function and insulin resistance (HOMA-B and HOMA-IR, respectively). Most of the phenotypes investigated for the subjects showed significant difference between the GDM patients group and the control group, (Table 1, P value < 0.0001 for all phenotypes except mean gestational week), which was to be expected because the phenotypes that showed the significant differences were associated with the diabetic condition. GDM patients were older, possessed higher BMI than the NGT women and clearly exhibited the clinical characteristics of T2DM, as shown in Table 1; in comparison to NGT women, GDM patients exhibited higher blood glucose levels and lower beta-cell function when insulin resistance was increased.
We first performed association analyses of the two genetic polymorphisms in GDM and non-GDM subjects to determine whether these polymorphisms were associated with a higher risk of developing GDM, which were previously found to be associated with T2DM (rs1387153 and rs10830963 on MTNR1B), with the risk of GDM (Table 2). The risk allele frequencies of both SNPS are also shown in Table 2. As a result, both SNPs showed significant associations with GDM in co-dominant, dominant, and recessive models. Co-dominant model exhibited lowest P value (0.00008, Pcor. = 0.0006, OR (95% CI) = 1.30 (1.06 - 1.66) and 0.00001, Pcor. = 0.00008, OR = 1.35 (1.18 - 1.54) for rs1387153 and rs10830963, respectively), and recessive model showed highest P value (0.002, OR = 1.42 (1.14 - 1.78) and 0.0001, OR = 1.54 (1.23 - 1.92) respectively for rs1387153 and rs10830963, respectively), while dominant model P value was in between the two (0.0007, OR (95% CI) = 1.44 (1.17 - 1.78) and 0.0006, OR = 1.46 (1.18 - 1.81) for rs1387153 and rs10830963, respectively). The risk allele for rs1387153 was T, and for rs10830963, it was G. P-value for Hardy-Weinberg equilibrium of subjects and controls were over 0.05 for both SNPs, indicating that the sample population is in Hardy-Weinberg equilibrium (data not shown). Also, there was a significantly high value of linkage disequilibrium (LD) between the two SNPs (|D'| = 0.89). The association results of haplotypes with the 2 SNPs are shown in Table 3 and 4.
We have also performed regression analysis with various diabetes-related phenotypes, including body mass index (BMI, analyzed controlling for age and the number of parities as covariate), AUC-G, HOMA-B, HOMA-IR, FPI and FPG, with age, BMI, and numbers of parities as covariates (Table 5). From the analyses, both SNPs showed significant associations for AUC-G, HOMA-B, and FPG with phenotypes among all subjects (Table 5). The rare alleles of the 2 SNPs were associated with bigger AUC-G, lower HOMA-B, and higher FPG, and these alleles were the risk alleles for GDM (T for rs1387153 and G for rs10830963). To see if a certain SNP was functional, we have performed conditional analyses for GDM and other phenotypes. The results are listed in Table 6. From the results, rs10830963 retained its signal, while the significance of rs1387153 was disappeared, which suggest that rs1387153 might have shown its signal because of its LD with rs10830963, although not clear in GDM-related phenotype analyses.
Discussion
In previous studies on the rs1387153 and rs10830963, researchers found strong associations between the two SNPs of MTNR1B and T2DM, and also with FPG levels, which is an important phenotype for diabetes. A study in European population, which included French and Danish among other nations, showed that rs1387153 was significantly associated with FPG level (P = 1.3 × 10-7, adjusted genome-wide P = 0.04) and T2DM (OR (95% CI) = 1.15(1.08-1.22), P = 6.3 × 10-5). Another study in European population found significant associations of rs10830963 with FPG (P = 3.2 × 10-50) and T2DM (OR (95% CI) = 1.09(1.05-1.12), P = 3.3 × 10-7). The Two SNPs were closely related with each other, as evidenced by the linkage disequilibrium test (|D'| = 0.89) in our study. Their haplotype analyses results showed that ht1 (C/C) and ht2 (T/G) were mostly tagged by rs10830963 and rs1387153, respectively (>92%). Therefore, ht1/ht2 showed similar associations with each SNP, respectively.
Here, we performed the association studies in Korean pregnant women and we found significant associations between the SNPs and GDM, with enough samples for high statistical power. It is well known that T2DM and GDM are closely related diseases, since they exhibit similar characteristics such as glucose intolerance. However, there had yet to be a study that looked into the association between the polymorphisms of MTNR1B and GDM, and our study confirmed the relations between the two. Also, we carried out regression analyses between the polymorphisms of MTNR1B and various phenotypes including FPG. Although both SNPs showed associations with GDM and FPG, our results suggested that the two genetic variants of MTNR1B were stronger risk factors for GDM in Korean population compared to the previous results for T2DM in European population (OR (95% CI) = 1.44 (1.17-1.78) for rs1387153 in dominant inheritance model and 1.46 (1.18 - 1.81) for rs10830963 in the present study and OR = 1.15 (1.08-1.22) for rs1387153 and OR = 1.09 (1.05-1.12) for rs10830963 in the two previous studies). We suspect that the genetic differences between GDM and T2DM and the population difference between Europeans and Asians could have contributed to this result. Previously, there have been a few cases where a gene associated with T2DM was not associated with GDM at all [20], or showed different effect sizes [21, 22], and our results suggest that MTNR1B affects T2DM and GDM in varying degrees as well.
Moreover, our results suggest that the two polymorphisms investigated are associated with beta-cell function (Table 5). Association between beta-cell function and MTNR1B was previously reported [18, 23], which shows that we were able to replicate the result in Korean population, strengthening the notion that MTNR1B polymorphisms are related with impaired beta-cell function. Recently, several groups of scientists have studied the association between the gene variants of MTNR1B and glucose tolerances. rs10830963 was found to be associated with FPG and decreased beta-cell function in a group of obese children, which is consistent with our finding [24]. Three independent studies of the MTNR1B genetic variants in Han Chinese subjects also found significant associations for increased FPG, impaired beta-cell function, glycated hemoglobin, and T2DM [18, 25, 26]. Furthermore, a study with European populations also found significant associations between variations of MTNR1B with BMI and FPG, but not with maturity-onset diabetes of the young (MODY) or T2DM [27]. Although some of their results do not agree with each other in the association with T2DM, these findings firmly back up the association between the SNPs in our study and FPG or impaired beta-cell function. On the other hand, we could not find any significant associations between insulin resistance (HOMA-IR) and the two genetic variants. Since our results are backed with high statistical power, this leads us to conclude that two genetic variants of MTNR1B may be associated with the disease by affecting glucose metabolism through impaired insulin secretion, as previously suggested by other studies with KCNQ1 genetic variants [8, 18, 28].
In addition, further conditional analyses results suggested that rs10830963 might be more likely functional in case/control analysis, although not clear in GDM-related phenotype analyses.
Although our results showed the significant associations with GDM and several diabetic characteristics, there are a couple of limitations. First, our study only concentrated on pregnant women among Korean population, so we cannot conclude that the MTNR1B gene variants are associated with FPG or impaired beta-cell function in all Korean population. Also, even though our study strongly suggests that the SNPs may also be associated with T2DM in Korean population, this is not confirmed yet. Any further studies on these two genetic variants in Korean population should concentrate on these parts.
Conclusions
The present study showed that two MTNR1B polymorphisms were associated with increased risk for GDM in Korean female population. Two polymorphisms rs1387153 and rs10830963 also showed significant associations with FPG and beta-cell function, but not with insulin resistance. Further conditional analyses results suggested that rs10830963 might be more likely functional in case/control analysis, although not clear in GDM-related phenotype analyses. The effective sizes found between the two polymorphisms and FPG was stronger compared to previous studies, which is possibly due to the genetic difference between European and Korean populations, or the difference between GDM and T2DM. Based on the current results, we suspect that these two polymorphisms will have significant associations with increased risk of GDM in other populations as well. Also, our discovery would be helpful for understanding of genetic etiology of GDM as well.
References
Cho YM, Kim TH, Lim S, Choi SH, Shin HD, Lee HK, Park KS, Jang HC: Type 2 diabetes-associated genetic variants discovered in the recent genome-wide association studies are related to gestational diabetes mellitus in the Korean population. Diabetologia. 2009, 52: 253-261. 10.1007/s00125-008-1196-4.
Metzger BE: Summary and recommendations of the Third International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes. 1991, 40 (Suppl 2): 197-201.
Amankwah K, Prentice R, Fleury F: The incidence of gestational diabetes. Obstet Gynecol. 1977, 49: 497-498.
Jovanovic L, Pettitt DJ: Gestational diabetes mellitus. Jama. 2001, 286: 2516-2518. 10.1001/jama.286.20.2516.
Buchanan TA, Xiang AH: Gestational diabetes mellitus. J Clin Invest. 2005, 115: 485-491.
Evans JC, Frayling TM, Cassell PG, Saker PJ, Hitman GA, Walker M, Levy JC, O'Rahilly S, Rao PV, Bennett AJ, Jones EC, Menzel S, Prestwich P, Simecek N, Wishart M, Dhillon R, Fletcher C, Millward A, Demaine A, Wilkin T, Horikawa Y, Cox NJ, Bell GI, Ellard S, McCarthy MI, Hattersley AT: Studies of association between the gene for calpain-10 and type 2 diabetes mellitus in the United Kingdom. Am J Hum Genet. 2001, 69: 544-552. 10.1086/323315.
Salonen JT, Uimari P, Aalto JM, Pirskanen M, Kaikkonen J, Todorova B, Hypponen J, Korhonen VP, Asikainen J, Devine C, Tuomainen TP, Luedemann J, Nauck M, Kerner W, Stephens RH, New JP, Ollier WE, Gibson JM, Payton A, Horan MA, Pendleton N, Mahoney W, Meyre D, Delplanque J, Froguel P, Luzzatto O, Yakir B, Darvasi A: Type 2 diabetes whole-genome association study in four populations: the DiaGen consortium. Am J Hum Genet. 2007, 81: 338-345. 10.1086/520599.
Prokopenko I, Langenberg C, Florez JC, Saxena R, Soranzo N, Thorleifsson G, Loos RJ, Manning AK, Jackson AU, Aulchenko Y, et al: Variants in MTNR1B influence fasting glucose levels. Nat Genet. 2009, 41: 77-81. 10.1038/ng.290.
Bouatia-Naji N, Bonnefond A, Cavalcanti-Proenca C, Sparso T, Holmkvist J, Marchand M, Delplanque J, Lobbens S, Rocheleau G, Durand E, et al: A variant near MTNR1B is associated with increased fasting plasma glucose levels and type 2 diabetes risk. Nat Genet. 2009, 41: 89-94. 10.1038/ng.277.
Peschke E, Frese T, Chankiewitz E, Peschke D, Preiss U, Schneyer U, Spessert R, Muhlbauer E: Diabetic Goto Kakizaki rats as well as type 2 diabetic patients show a decreased diurnal serum melatonin level and an increased pancreatic melatonin-receptor status. J Pineal Res. 2006, 40: 135-143. 10.1111/j.1600-079X.2005.00287.x.
Lee YH, Kang ES, Kim SH, Han SJ, Kim CH, Kim HJ, Ahn CW, Cha BS, Nam M, Nam CM, Lee HC: Association between polymorphisms in SLC30A8, HHEX, CDKN2A/B, IGF2BP2, FTO, WFS1, CDKAL1, KCNQ1 and type 2 diabetes in the Korean population. J Hum Genet. 2008, 53: 991-998. 10.1007/s10038-008-0341-8.
Carpenter MW, Coustan DR: Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol. 1982, 144: 768-773.
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC: Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985, 28: 412-419. 10.1007/BF00280883.
Krzyzanowska K, Zemany L, Krugluger W, Schernthaner GH, Mittermayer F, Schnack C, Rahman R, Brix J, Kahn BB, Schernthaner G: Serum concentrations of retinol-binding protein 4 in women with and without gestational diabetes. Diabetologia. 2008, 51: 1115-1122. 10.1007/s00125-008-1009-9.
Livak KJ: Allelic discrimination using fluorogenic probes and the 5' nuclease assay. Genet Anal. 1999, 14: 143-149.
Barrett JC, Fry B, Maller J, Daly MJ: Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005, 21: 263-265. 10.1093/bioinformatics/bth457.
Menashe I, Rosenberg PS, Chen BE: PGA: power calculator for case-control genetic association analyses. BMC Genet. 2008, 9: 36-
Zhou Q, Zhang K, Li W, Liu JT, Hong J, Qin SW, Ping F, Sun ML, Nie M: Association of KCNQ1 gene polymorphism with gestational diabetes mellitus in a Chinese population. Diabetologia. 2009, 52: 2466-2468. 10.1007/s00125-009-1500-y.
Stephens M, Smith NJ, Donnelly P: A new statistical method for haplotype reconstruction from population data. Am J Hum Genet. 2001, 68: 978-989. 10.1086/319501.
Shaat N, Lernmark A, Karlsson E, Ivarsson S, Parikh H, Berntorp K, Groop L: A variant in the transcription factor 7-like 2 (TCF7L2) gene is associated with an increased risk of gestational diabetes mellitus. Diabetologia. 2007, 50: 972-979. 10.1007/s00125-007-0623-2.
Grant SF, Thorleifsson G, Reynisdottir I, Benediktsson R, Manolescu A, Sainz J, Helgason A, Stefansson H, Emilsson V, Helgadottir A, Styrkarsdottir U, Magnusson KP, Walters GB, Palsdottir E, Jonsdottir T, Gudmundsdottir T, Gylfason A, Saemundsdottir J, Wilensky RL, Reilly MP, Rader DJ, Bagger Y, Christiansen C, Gudnason V, Sigurdsson G, Thorsteinsdottir U, Gulcher JR, Kong A, Stefansson K: Variant of transcription factor 7-like 2 (TCF7L2) gene confers risk of type 2 diabetes. Nat Genet. 2006, 38: 320-323. 10.1038/ng1732.
Leipold H, Knoefler M, Gruber C, Huber A, Haslinger P, Worda C: Peroxisome proliferator-activated receptor gamma coactivator-1alpha gene variations are not associated with gestational diabetes mellitus. J Soc Gynecol Investig. 2006, 13: 104-107. 10.1016/j.jsgi.2005.12.004.
Staiger H, Machicao F, Schafer SA, Kirchhoff K, Kantartzis K, Guthoff M, Silbernagel G, Stefan N, Haring HU, Fritsche A: Polymorphisms within the novel type 2 diabetes risk locus MTNR1B determine beta-cell function. PLoS One. 2008, 3: e3962-10.1371/journal.pone.0003962.
Holzapfel C, Siegrist M, Rank M, Langhof H, Grallert H, Baumert J, Irimie C, Klopp N, Wolfarth B, Illig T, Hauner H, Halle M: Association of a MTNR1B gene variant with fasting glucose and HOMA-B in children and adolescents with high BMI-SDS. Eur J Endocrinol. 2010
Kan MY, Zhou DZ, Zhang D, Zhang Z, Chen Z, Yang YF, Guo XZ, Xu H, He L, Liu Y: Two susceptible diabetogenic variants near/in MTNR1B are associated with fasting plasma glucose in a Han Chinese cohort. Diabet Med. 2010, 27: 598-602. 10.1111/j.1464-5491.2010.02975.x.
Tam CH, Ho JS, Wang Y, Lee HM, Lam VK, Germer S, Martin M, So WY, Ma RC, Chan JC, Ng MC: Common polymorphisms in MTNR1B, G6PC2 and GCK are associated with increased fasting plasma glucose and impaired beta-cell function in Chinese subjects. PLoS One. 2010, 5: e11428-10.1371/journal.pone.0011428.
Andersson EA, Holst B, Sparso T, Grarup N, Banasik K, Holmkvist J, Jorgensen T, Borch-Johnsen K, Egerod KL, Lauritzen T, Sorensen TI, Bonnefond A, Meyre D, Froguel P, Schwartz TW, Pedersen O, Hansen T: The MTNR1B G24E variant associates with BMI and fasting plasma glucose in the general population in studies of 22,142 Europeans. Diabetes. 2010
Mussig K, Staiger H, Machicao F, Kirchhoff K, Guthoff M, Schafer SA, Kantartzis K, Silbernagel G, Stefan N, Holst JJ, Gallwitz B, Haring HU, Fritsche A: Association of type 2 diabetes candidate polymorphisms in KCNQ1 with incretin and insulin secretion. Diabetes. 2009, 58: 1715-1720. 10.2337/db08-1589.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2350/12/82/prepub
Acknowledgements
This study was supported by a grant from the Korean Research Foundation in Korea (R04-2008-000-10078-0), Korea Science and Engineering Foundation (KOSEF) funded by the Korea government (MEST) (No. 2009-0080157), and the Korea Health 21 R&D Project, Ministry of Health & Welfare, Republic of Korea (Grant No. A050463).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
JYK and HSC developed tables/figures, and wrote the manuscript. SHB, SWL, SMP, MHK, JHC, JSC, MYK, JHY, and DHC helped recruiting subjects, conducted experiments, and collected data. HSC and BLP analyzed data by performing statistical analysis. HDS and SHK managed all of the study and helped to draft the manuscript. All authors have read and approved the final manuscript.
Jason Y Kim, Hyun Sub Cheong contributed equally to this work.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Kim, J.Y., Cheong, H.S., Park, BL. et al. Melatonin receptor 1 B polymorphisms associated with the risk of gestational diabetes mellitus. BMC Med Genet 12, 82 (2011). https://doi.org/10.1186/1471-2350-12-82
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2350-12-82