Abstract
Aims
To quantitatively analyze and compare the differences in retinal neurovascular units (NVUs) between healthy individuals and patients with type 2 diabetes mellitus (DM) by optical coherence tomography (OCT) and optical coherence tomography angiography (OCTA) techniques and to determine the value of this technique for the early diagnosis of retinal neurovascular damage in patients with diabetes mellitus without retinopathy (NDR).
Methods
This observational case‒control study was conducted from July 1, 2022, to November 30, 2022, at the outpatient ophthalmology clinic of the Affiliated Hospital of Shandong University of Traditional Chinese Medicine. All subjects underwent baseline data entry and mean thickness of the peripapillary retinal nerve fiber layer (pRNFL), the thickness of each retinal layer in the macula 3 × 3 mm, and vascular density (VD) examination.
Results
The study included 35 healthy individuals and 48 patients with DM. The retinal VD as well as partial pRNFL, macular nerve fiber layer (NFL), and macular ganglion cell layer (GCL) thickness in DM patients exhibited significantly lower VD in the DM group than in the control group (p < 0.05). Age and disease duration of DM patients showed a negative trend with pRNFL thickness, macular NFL thickness, macular GCL thickness, and VD. However, a positive trend was observed between DM duration and partial inner nuclear layer (INL) thickness. Moreover, there was a positive correlation between macular NFL and GCL thickness and VD for the most part, while a negative correlation was shown between INL temporal thickness and DVC-VD. pRNFL-TI and GCL-superior thickness were screened as two variables in the analysis of the predictors of retinal damage in DM according to the presence or absence of DM. The AUCs were 0.765 and 0.673, respectively. By combining the two indicators for diagnosis, the model predicted prognosis with an AUC of 0.831. In the analysis of retinal damage indicators associated with the duration of DM, after regression logistic analysis according to the duration of DM within 5 years and more than 5 years, the model incorporated two indicators, DVC-VD and pRNFL-N thickness, and the AUCs were 0.764 and 0.852, respectively. Combining the two indicators for diagnosis, the AUC reached 0.925.
Conclusions
Retinal NVU may have been compromised in patients with DM without retinopathy. Basic clinical information and rapid noninvasive OCT and OCTA techniques are useful for the quantitative assessment of retinal NVU prognosis in patients with DM without retinopathy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The prevalence of diabetes mellitus (DM), a serious, long-term disease, continues to rise rapidly worldwide [1]. According to the International Diabetes Federation, there are nearly 500 million people with DM worldwide, and 579 million people are expected to have DM in 2030, a number that will increase to 700 million in 2045 [2]. Diabetic retinopathy (DR), a major complication of DM, occurs in approximately 30%–40% of diabetic patients. Globally, more than 100 million people live with DR, and it is the leading cause of vision loss in middle-aged and older adults [3, 4]. DR has been considered a microvascular disease. The earliest response of the retinal vasculature to hyperglycemia is vasodilation and altered blood flow, triggering pericyte loss, endothelial cell apoptosis, and basement membrane thickening [5, 6]. Clinical manifestations such as retinal hemorrhages and microaneurysms are present when the damage to the retina is irreversible. Recent studies have shown that retinal neurodegeneration may precede microaneurysms [7]. Therefore, the understanding of DR has shifted from simple microvascular injury to the imbalance of neurovascular unit (NVU) injury and its coupling mechanism [8]. The structure of the NVU consists of retinal neurons, glial cells, and the retinal microvascular system, which are distributed in the corresponding 10 layers of the retina. Early detection and diagnosis have become the goals of many researchers, and because of its insidious onset, there is an urgent need for improved means of monitoring the progression of DR.
For many years, fluorescein angiography (FA) has been used as the reference method for visualizing retinal circulation. This 2D invasive technique, in addition to the potential risk of intravenous injection, does not allow for precise stratification of the retina and imaging of the blood vessels specifically around the optic disk [9, 10]. In recent years, the introduction of optical coherence tomography (OCT) has revolutionized the visualization and quantification of retinal stratification [10]. Optical coherence tomography angiography (OCTA) is a new noninvasive imaging modality that allows visualization of the microvasculature of the retina and choroid in different layers, allowing easy measurement of microvascular changes in the peripapillary and macular regions [11]. This technique allows easy and accurate measurement of retinal changes in diabetic patients without DR. Previous studies have mostly focused on microcirculation in the macula or optic disk area or on changes in the thickness of the retinal nerve fiber layer (NFL) [12,13,14]. In contrast, studies focusing on the NVU using OCT or OCTA techniques are limited. We know that the NVU is mainly distributed in the retinal NFL to the inner nuclear layer (INL). The quantitative analysis of the thickness of the main distribution levels of the NVU in the retina and blood flow densitometry allow a comprehensive assessment of the imbalance of the coupling mechanism of the retinal NVU in diabetic patients without DR.
In this observational case‒control study, we quantified OCT and OCTA indices of the optic disk and macula in type 2 diabetic patients without DR and healthy subjects, respectively, to assess the interconnection between retinal nerve, glial cell, and microvascular damage in early diabetic patients and to provide diagnostic value for the prevention of DR.
Methods
Subjects
This study was approved by the Ethics Committee of the Affiliated Hospital of Shandong University of Traditional Chinese Medicine, Approval No. (2021) Ethics Audit No. (013)-XY, in accordance with the Declaration of Helsinki guidelines. Informed consent was obtained from all participants. This cross-sectional single-center study involved 48 patients diagnosed with type 2 diabetes using standard methods and 35 healthy individuals as controls. The participants underwent routine ophthalmologic examinations, including best-corrected visual acuity (BCVA), slit lamp microscopy, direct ophthalmoscopy, and noncontact tonometer for intraocular pressure (intraocular pressure (IOP). Age, sex, highest fasting blood glucose concentration, and duration of diabetes were also recorded. The BCVA was checked using the international standard visual acuity chart, which was converted into logarithm of the minimal angle of resolution (logMAR) visual acuity for statistical analysis. Two independent investigators (BHL and WWL) reviewed all medical records and fundus conditions to determine the status of diabetic retinopathy and excluded patients with abnormal fundus findings associated with diabetic retinopathy, such as retinal hemorrhages, exudates, and cotton wool spots.
Inclusion criteria: Age ≥ 18 years; IOP ≤ 21 mmHg; spherical equivalent (SE) between + 3.00 (diopters) D and -3.00 D. Met the definition of type 2 diabetes mellitus with a randomized blood glucose concentration of ≥ 200 mg/dL (11.1 mmol/L) according to the criteria recommended by the World Health Organization dL (11.1 mmol/L) or a fasting glucose concentration of ≥ 126 mg/dL (7.0 mmol/L) or a plasma glucose concentration ≥ 200 mg/dL (11.1 mmol/L) 2 h after a 75 g oral glucose load [15].
Exclusion criteria: diagnosed diabetic retinopathy, high myopia, glaucoma, anterior retina, hypertensive retinopathy, macular degeneration, retinal hemorrhage, retinal vascular obstruction or uveitis; intraocular surgery such as vitrectomy, cataract surgery, glaucoma surgery, etc.; diseases that affect the quality of OCT and OCTA scans, such as nystagmus, ulcerative keratitis, cataract, etc.
OCT and OCTA
All subjects underwent OCT (Spectralis, Heidelberg Engineering Gmbh, Germany) for corresponding measurements of the macula and optic disk area, with images taken in natural light. Patients were seated, and all subjects were fully dilated with 0.5% tropicamide before the examination, with the lower jaw placed in the jaw rest and the forehead against the frontal rest. The height of the jaw rest was adjusted, and the patient was instructed to look inwardly at the visual standard. All scans were performed by the same experienced examiner (LWW), and all scans were reviewed individually by two researchers (HBL and CMR).
pRNFL thickness measurement
Using OCT mode with a light source at 870 nm, the peripapillary retinal nerve fiber layer (pRNFL) was measured within 3.7 mm of the peripapillary area around the optic disk. A circular scan with a diameter of 3.7 mm and a depth of 25 mm was employed with the software that comes with the device. For the average thickness, the software is automatically divided into six sections, namely supranasal (Superonasal, NS), nasal (Nasal, N), inferior nasal (Inferonasal, NI), superior temporal (Superotemporal, TS), lateral temporal (Temporal, T), and inferior temporal (Inferotemporal, TI). The mean thickness of each part of the pRNFL was recorded. See Fig. 1.
Circumferential scan of the peripapillary retinal nerve fiber layer. The illustration shows an OCT scan of the peripapillary retinal nerve fiber layer of the right eye of a patient. The average thickness of the supranasal (NS), nasal (N), inferonasal (NI), superotemporal (TS), temporal (T) and inferotemporal (TI) subdivisions were recorded on the figure
Average retinal thickness and vascular density measurement in each layer of the macula
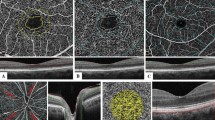
The OCTA mode was used to record the average thickness of the nerve fiber layer (NFL), ganglion cell layer (GCL), inner plexiform layer (IPL), and inner nuclear layer (INL) in the central region of the retina at 3 × 3 mm, above, below, nasal, temporal and central, using the central macular recess as the center of the scan, with a 3 mm × 3 mm (10°*10°) transverse resolution of 5.7 µm/pixel (512A scan * 512B scan); cell layer (GCL), inner plexiform layer (IPL) and inner nuclear layer (INL). Vascular images of the superficial vascular complex, deep vascular complex and avascular complex were also preserved in 3 × 3 mm OCTA mode scans. Data collection criteria: complete data without loss in the OCTA scan window, uniform color density, no signal interference, OCTA quality intensity ≥ 30. See Fig. 2.
OCTA scans of the macula. Image A shows a 3*3 mm pattern OCTA pattern scan of the right eye of a patient showing the average thickness of the ganglion cell layer in the superior, inferior, nasal, temporal and central subdivisions. Image B documents the superficial vascular complex, deep vascular complex and B-mode images of the superficial vascular complex, deep vascular complex and avascular complex
Terminology
Superficial vascular complex (SVC): inner limiting membrane (ILM) to 10 μm above the IPL.
Deep vascular complex (DVC): extends from 10 μm above the IPL to 10 μm below the outer plexiform layer (OPL).
Avascular complex (AC): 10 μm extension below the OPL to the Bruch membrane.
VD represents the ratio of blood flow area to total area scanned using OCTA.
Statistical analysis
All statistical analyses were performed using the statistical software SPSS version 25.0. Continuous variables are expressed as the mean ± standard deviation (\(\overline{x}\pm \mathrm{S }\)), and categorical variables are summarized as frequencies and percentages. Vessel density was analyzed using ImageJ software. Baseline characteristics of the different patient groups were summarized and compared according to the distribution of each variable using Fisher's exact test and t test. Between-group differences in retinal thickness and vessel density were compared using multiple linear regression or the covariance method corrected for the variable of age. Pearson correlation analysis was used to analyze the two correlations between baseline data and retinal thickness and vessel density. Because age was considered to significantly differ between the two groups, we verified the correlation between retinal thickness and vessel density by correcting for the variable of age using partial correlation. The presence or absence of diabetes mellitus was used as a dichotomous variable grouping to perform univariate and multivariate logistic regression analyses to screen for possible correlates of diabetic retinal damage. The variables that entered the model and the predicted probability PRE_1 of the new variables outputting the joint diagnosis when the logistic regression model was established in this study were used to plot the receiver operator characteristic (ROC) curve to determine the joint diagnostic index of diabetic retinal damage. The duration of disease among diabetic patients was grouped as dichotomous variables within 5 years and more than 5 years, and univariate and multivariate logistic regression analyses were performed to screen for retinal damage factors associated with the duration of diabetes. The variables entered into the model and the predicted probability PRE_2 of the new variables outputting the joint diagnosis when the logistic regression model was established in this study were used to plot the ROC curve to determine the joint diagnostic index of retinal damage factors associated with the duration of diabetes mellitus. All statistical analyses were defined as statistically significant at P < 0.05.
Results
Demographic and clinical data
Ninety-six eyes of 48 diabetic patients and 70 eyes of 35 healthy individuals were evaluated in this study. After rigorous screening, the final OCT thickness examination of the pRNFL included both eyes of all examinees. The OCTA examination of the macular area randomly selected a single eye of each examinee, excluding those of substandard quality, and ultimately evaluated 47 eyes of 47 diabetic patients and 34 eyes of 34 healthy individuals. The mean age of the diabetic patients was 57.58 ± 8.19 years; 17 patients were male and 31 patients were female; all DM patients were DR-free; all DM patients had a mean disease of 8.13 ± 5.73 years and a mean maximum fasting glucose of 13.27 ± 4.12 mmol/L. The mean age of the healthy individuals was 51.14 ± 14.13 years; 14 cases were male, and 21 cases were female. The demographic and clinical characteristics of all study participants are summarized in Table 1. There were no significant differences between the groups in terms of sex, BCVA, or IOP (p < 0.05). Healthy individuals and diabetic patients showed significant differences in terms of age (p = 0.019).
Comparison of pRNFL thickness, VD, and average retinal thickness in the macular area
Statistical analysis of variance showed that after correcting for age as a baseline variable, the pRNFL thickness was significantly lower in the three subdivisions of TI, NS, and NI in the diabetic group than in normal subjects (p < 0.05, see Table 2). In contrast, the NFL-Inferior, NFL-Center, GCL-Superior, GCL-Temporal, and GCL-Inferior showed significantly lower retinal thickness in the diabetic group than in the normal group in all layers of the macula (p < 0.05, see Table 3). The vascular density in the macular region, whether in the superficial vascular complex, deep vascular complex or avascular complex, was significantly lower in the diabetic group than in the normal group (p < 0.05, see Table 3).
Correlation of pRNFL thickness or VD with basic clinical data
Correlation analysis of pRNFL thickness with baseline data showed that pRNFL-NI thickness was significantly negatively correlated with age (r = − 0.279, p = 0.011); pRNFL-TI, pRNFL-N, and pRNFL-NI thickness were significantly negatively correlated with DM duration (r = − 0.595, p = 0.000; r = − 0.615, p = 0.000 and r = − 0.555, p = 0.000); see Table 4. Correlation analysis of vessel density with baseline data showed a significant negative correlation between SVC-VD and age (r = − 0.299, p = 0.007); SVC-VD, DVC-VD, and duration of DM also showed a significant negative correlation (r = − 0.578, p = 0.000 and r = − 0.440, p = 0.003); see Table 5.
Correlation of macular VD with the mean thickness of each retinal layer or the duration of DM
Correlation analysis after correcting for age variables showed that most of the NFL and the subdivisional thickness of the GCL in the macula showed a positive correlation trend with vascular density, and IPL-Superior thickness showed the same positive correlation trend with SVC-VD. NFL-Superior, NFL-Nasal, NFL-Inferior, and NFL-Center thicknesses showed a negative correlation with the duration of DM, GCL-Inferior and GCL-Center thicknesses showed a negative correlation with the duration of DM, and IPL-Superior thickness showed a negative correlation with the duration of DM. Superior thickness showed a negative correlation with the duration of DM. However, a positive trend was observed between IPL-Center, INL-Temporal, and INL-Inferior thicknesses and the duration of DM. All statistics are presented in Table 6.
Indicators associated with diabetic retinal damage
Age variables with statistically significant differences in the analysis of baseline correlates were included in the subsequent regression analysis with all OCT and OCTA results. Logistic regression analysis was performed according to the presence or absence of diabetes mellitus; after selecting variables with significant differences for univariate regression analysis, those with significantly different univariate variables were then subjected to multiple regression analysis using the forward step method, and the results of the predictive model parameter estimation and testing are shown in Table 7. The 2 independent variables of retinal thickness included in the model (pRNFL-TI, GCL- Superior) were statistically significant (p < 0.05), and the resulting prediction model was tested by the Hosmer‒Lemeshow goodness-of-fit test χ2 = 29.607, p = 0.000, -2 log likelihood = 80.587, and the prediction accuracy of the model was 74.1%. The logistic regression equation can be obtained as Logit (p) = -22.618 + 0.086 × pRNFL-TI + 0.190 × GCL-Superior.
The ROC curves were plotted by combining the 2 variables that entered the model (pRNFL-TI, GCL-Superior thickness) and the predicted probability of the new variable PRE_1 of the joint diagnosis that was output when the logistic regression model was established in this study, as shown in Fig. 3. The area under the curve was 0.765, 0.673, and 0.831, respectively.
Indicators of retinal damage related to the duration of diabetes
The continuous variable DM duration was transformed into a dichotomous variable according to the duration of diabetes mellitus within 5 years and more than 5 years, DM duration ≤ 5 years = 0, DM duration > 5 years = 1; variables with significant correlation with the duration of diabetes mellitus in Age, Tables 4, 5, 6 were selected for univariate regression After logistic analysis, the respective significance was collected, and the univariate variables that were significantly different were then subjected to multiple regression analysis using the forward step method, and the results of the prediction model parameter estimation and testing are shown in Table 8. The 2 independent variables (DVC-VD and pRNFL-N thickness) incorporated in the model were statistically significant (p < 0.05), and the resulting prediction model was validated by the Hosmer- Lemeshow goodness-of-fit test χ2 = 33.320, p = 0.000, -2 log likelihood = 31.645, and the prediction accuracy of the model was 85.1%. The logistic regression equation can be obtained as Logit (p) = − 20.294 + 0.296 × DVC-VD + 0.196 × pRNFL-N.
The ROC curves were plotted by taking the 2 variables that entered the model (DVC-VD and pRNFL-N thickness) and the predicted probability of the new variable PRE_2 of the joint diagnosis of the output when the logistic regression model was built in this study (see Fig. 4). The areas under the curves were 0.764, 0.852, and 0.925, respectively.
Discussion
In this study, we identified several baseline characteristics of DM patients, OCT, and quantitative indicators of their OCAT that may reflect the risk or diagnostic value of developing DR in DM patients. Most studies on early changes in preclinical DR are based on the genetic and protein levels, while clinical changes are unknown [16,17,18]. We know that the main components of the retinal NVU include neurons, glial cells, and the retinal microvascular system. There are five types of retinal neurons: photoreceptors, horizontal cells, bipolar cells, amacrine cells, and ganglion cells. Photoreceptors are mainly located in the outer nuclear layer (ONL). The cell bodies of horizontal, bipolar, and anaplastic cells are mainly located in the INL. Retinal ganglion cells (RGCs) are distributed in the GCL [19]. The glial component consists of macroglia (astrocytes and Müller cells), microglia, and oligodendrocytes, which are the interface between neurons and the vascular system, regulate functional communication between them and are present between the inner boundary membrane (ILM) and the outer membrane (OLM) [20]. However, retinal microvessels are distributed from the NFL to the INL and are not present in the ONL. In this study, we looked at the functional impairment of the retinal NVU in DM patients, so we investigated the quantitative analysis of OCT and OCTA from the retinal NFL to the INL [8, 21].
An important finding of this study was that retinal VD, NFL, and GCL thickness were lower in DM patients than in healthy subjects, even in the absence of DR (Tables 2 and 3). Correlation analysis of baseline information with retinal indicators revealed (Tables 4, 5, 6) that there was no significant correlation between retinal VD and maximum fasting glucose, although blood glucose level was the most important indicator for the diagnosis of diabetes. pRNFL-NI thickness was significantly and negatively correlated with age, and pRNFL-TI, pRNFL-N, and pRNFL- NI thickness was significantly and negatively correlated with the duration of DM disease. SVC-VD showed a significant negative correlation with age, and similarly, SVC-VD and DVC-VD showed a significant negative correlation with the duration of DM. The macula also showed a negative trend of correlation between the thickness of some NFL subdivisions and the duration of DM. This suggests that age may be a risk factor for the development of DR, and the older the age, the greater the risk of retinal vascular damage. Similarly, the duration of DM disease is also a risk factor for the development of DR, and the longer the duration of DM disease is, the greater the risk of nerve fiber and vascular damage in the retina. This finding is consistent with some previous studies [22]. Our updated study showed a negative correlation between the thickness of the inferior and center subdivisions of the GCL and the duration of DM disease, suggesting that RGCs are damaged as the duration of DM disease increases. The rat model of DR by Wang QC et al. similarly demonstrated that hyperglycemia induces the death of RGCs [23]. To our surprise, INL-temporal and INL-inferior thicknesses showed a positive trend in correlation with the course of DM. We retrieved two articles showing increased IPL and INL thickness in patients with nonproliferative DR (NPDR) compared to those with NDR and attributed this to increased INL thickness due to early microglial activation and aggregation [24]. Bandello F et al. similarly found that the increase in retinal thickness in NPDR patients compared to healthy individuals was located mainly in the INL and extended to the adjacent retinal layers [25]. We know that the INL contains the nuclei of Müller cells and most microglia in addition to the cell bodies of horizontal, bipolar, and anaplastic cells for cell and animal experiments revealed that under high sugar conditions, Müller and microglial cells become active and induce oxidative stress and inflammation [26, 27]. Activation, value-added, edema, and hypertrophy of glial cells in the retinal NVU may be the main causes of INL thickening [28].
In the correlation analysis between retinal thickness and VD in the macula (Table 6), most of the subdivisions of the NFL and GCL in the macula showed a positive trend of correlation with vascular density; however, most of the subdivisions of the INL showed a negative trend of correlation with vascular density. Damage to nerve fibers and RGCs can cause microvascular damage, and the activation, value-added, edema of glial cells, as well as the oxidative stress and inflammation triggered by them, can also lead to alterations in microvascular canal diameter and blood flow. Of course, this effect may also be bidirectional. The physiological demands of the retinal neurons determine the filling of the vessels and the changes in their lumen diameter [29]. The retinal microvasculature provides nutrients and oxygen to the NVU and excretes waste products to meet the high demand for oxygen consumption by the retina, a metabolically active neurovascular tissue [30, 31]. Nerve and glial cell lesions can affect the microvasculature, and microvascular lesions can similarly involve the glia [8].
In the analysis of the predictors of retinal damage in DM, logistic regression analysis was performed according to the presence or absence of DM, and two variables, pRNFL-TI and GCL-Superior thickness, were screened. The AUCs of the 2 variables for the prediction of retinal damage in DM patients differed (0.765 and 0.673, respectively), and the differences between the AUCs of each variable and the predictive ability of the model were statistically significant (p < 0.05). The AUC of the model predicting prognosis reached 0.831 when the 2 indicators were combined for diagnosis. According to the judgment criteria of predictive efficacy, AUC > 0.8, the judgment efficacy is excellent, which is more advantageous than applying each variable alone to predict retinal damage in DM patients, suggesting that clinicians can pay attention to the early prevention of retinal damage in DM patients based on the thickness changes in the pRNFL and GCL.
In the analysis of retinal damage indicators associated with the duration of DM disease, the model incorporating DVC-VD and pRNFL-N thickness was statistically significant (p < 0.05) after regression logistic analysis according to the duration of DM disease within 5 years and more than 5 years. Combining the two indices for diagnosis, the model predicted an AUC of 0.925, which is excellent for judgmental efficacy and more advantageous than applying each variable alone for predicting retinal damage in patients with long-term DM, suggesting that the duration of DM disease is a nonnegligible factor for DR. Clinicians should thus pay special attention to the nerve fibers and superficial microvessels in the retina of patients with DM over 5 years.
Two of the three indicators in the model analysis with the presence or absence of DM were based on the pRNFL and one on the GCL, and it can be seen that VD did not appear significantly different. However, in the prediction model categorized by 5 years of DM disease, DVC-VD appeared, still with pRNFL thickness. This indicates that the neurological changes around the optic disk may occur earlier in the development of DR, while most of our clinical focus is only on retinal damage in the macula. We should note that the axonal density of the optic papilla is higher than that of the macula. In the early stages, retinal damage may be seen mainly at the neural level, such as RGCs and glial cells. The microvascular changes only appear slowly as the disease continues to extend, which is similar to the findings of Zhang et al. [14]. The process of collecting indicators such as DM disease duration, OCT, and OCTA is relatively simple, and these indicators can be judged in a short period of time, which is suitable to be applied in the disease prognosis assessment of DM patients to indicate the possible course of their disease. Early and effective interventions for patients may have a greater chance at saving visual impairment.
Conclusion
Chronic hyperglycemia leads to hypoxia and causes retinal inflammation, factors that may contribute to impaired retinal NVU and its coupling mechanism imbalance in diabetic patients. Basic clinical information and rapid noninvasive OCT and OCTA techniques are useful for the quantitative assessment of retinal NVU prognosis in DM patients without retinopathy.
Abbreviations
- DM:
-
Diabetes mellitus
- DR:
-
Diabetic retinopathy
- NDR:
-
Patients with diabetes without clinically detectable retinopathy
- OCT:
-
Optical coherence tomography
- OCTA:
-
Optical coherence tomography angiography
- NVU:
-
Neurovascular unit
- pRNFL:
-
Peripapillary retinal nerve fiber layer
- NFL:
-
Nerve fiber layer
- GCL:
-
Ganglion cell layers
- IPL:
-
Inner plexiform layer
- INL:
-
Inner nuclear layer
- BCVA:
-
Best-corrected visual acuity
- IOP:
-
Intraocular pressure
- logMAR:
-
Logarithm of the minimal angle of resolution
- SVC:
-
Superficial vascular complex
- DVC:
-
Deep vascular complex
- AC:
-
Avascular complex
- VD:
-
Vascular density
- ROC:
-
Receiver operating characteristic
- AUC:
-
The area under the ROC
- SD:
-
Standard deviation
References
Vujosevic S, Aldington SJ, Silva P et al (2020) Screening for diabetic retinopathy: new perspectives and challenges. Lancet Diabetes Endocrinol 8(4):337–347
Saeedi P, Petersohn I, Salpea P et al (2019) Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res Clin Pract 157:107843
Tan TE, Wong TY (2022) Diabetic retinopathy: looking forward to 2030. Front Endocrinol (Lausanne) 13:1077669
Ruta LM, Magliano DJ, Lemesurier R et al (2013) Prevalence of diabetic retinopathy in type 2 diabetes in developing and developed countries. Diabet Med 30(4):387–398
Beltramo E, Porta M (2013) Pericyte loss in diabetic retinopathy: mechanisms and consequences. Curr Med Chem 20:3218–3225
Bek T (2017) Diameter changes of retinal vessels in diabetic retinopathy. Curr Diab Rep 17(10):82
Duh EJ, Sun JK, Stitt AW (2017) Diabetic retinopathy: current understanding, mechanisms, and treatment strategies. JCI Insight 2(14):e93751
Li B, Ning B, Yang F, Guo C (2022) Nerve growth factor promotes retinal neurovascular unit repair: a review. Curr Eye Res 47:1095–1105
Mendes L, Marques IP, Cunha-Vaz J (2021) Comparison of different metrics for the identification of vascular changes in diabetic retinopathy using OCTA. Front Neurosci 15:755730
Shin YI, Nam KY, Lee SE et al (2019) Peripapillary microvasculature in patients with diabetes mellitus: an optical coherence tomography angiography study. Sci Rep 9:15814
Li Z, Alzogool M, Xiao J et al (2018) Optical coherence tomography angiography findings of neurovascular changes in type 2 diabetes mellitus patients without clinical diabetic retinopathy. Acta Diabetol 55:1075–1082
Yang D, Cao D, Huang Z et al (2019) Macular capillary perfusion in chinese patients with diabetic retinopathy obtained with optical coherence tomography angiography. Ophthalmic Surg Lasers Imag Retin 50:e88–e95
Kim K, Kim ES, Kim DG, Yu SY (2019) Progressive retinal neurodegeneration and microvascular change in diabetic retinopathy: longitudinal study using OCT angiography. Acta Diabetol 56:1275–1282
Zhang M, Jia F, Li N et al (2021) Quantitative analysis of the RPC vessel density and the RNFL thickness in patients with type 2 diabetes mellitus by using OCT angiography. Ophthalmic Res 64:951–959
Xu J, Wei WB, Yuan MX et al (2012) Prevalence and risk factors for diabetic retinopathy: the Beijing communities diabetes study 6. Retina 32:322–329
Garcia TB, Hollborn M, Bringmann A (2017) Expression and signaling of NGF in the healthy and injured retina. Cytokine Growth Factor Rev 34:43–57
Mysona BA, Matragoon S, Stephens M et al (2015) Imbalance of the nerve growth factor and its precursor as a potential biomarker for diabetic retinopathy. Biomed Res Int 2015:571456
Mysona BA, Al-Gayyar MM, Matragoon S et al (2013) Modulation of p75(NTR) prevents diabetes- and proNGF-induced retinal inflammation and blood-retina barrier breakdown in mice and rats. Diabetologia 56:2329–2339
Meng C, Gu C, He S et al (2021) Pyroptosis in the retinal neurovascular unit: new insights into diabetic retinopathy. Front Immunol 12:763092
Bilimoria PM, Stevens B (2015) Microglia function during brain development: new insights from animal models. Brain Res 1617:7–17
Tawfik A, Samra YA, Elsherbiny NM, Al-Shabrawey M (2020) Implication of hyperhomocysteinemia in blood retinal barrier (BRB) dysfunction. Biomolecules 10(8):1119
Li X, Yu Y, Liu X et al (2021) Quantitative analysis of retinal vessel density and thickness changes in diabetes mellitus evaluated using optical coherence tomography angiography: a cross-sectional study. BMC Ophthalmol 21:259
Wang QC, Sheng W, Yi CJ, Lv H, Cheng B (2020) Retrobulbarly injecting nerve growth factor attenuates visual impairment in streptozotocin-induced diabetes rats. Int Ophthalmol 40:3501–3511
Vujosevic S, Midena E (2013) Retinal layers changes in human preclinical and early clinical diabetic retinopathy support early retinal neuronal and Muller cells alterations. J Diabetes Res 2013:905058
Bandello F, Tejerina AN, Vujosevic S et al (2015) Retinal layer location of increased retinal thickness in eyes with subclinical and clinical macular edema in diabetes type 2. Ophthalmic Res 54:112–117
Tu Y, Song E, Wang Z et al (2021) Melatonin attenuates oxidative stress and inflammation of Muller cells in diabetic retinopathy via activating the Sirt1 pathway. Biomed Pharmacother 137:111274
Krishnan G, Chatterjee N (2012) Endocannabinoids alleviate proinflammatory conditions by modulating innate immune response in muller glia during inflammation. Glia 60:1629–1645
Rubsam A, Parikh S, Fort PE (2018) Role of inflammation in diabetic retinopathy. Int J Mol Sci 19(4):942
Kowianski P, Lietzau G, Steliga A et al (2013) The astrocytic contribution to neurovascular coupling–still more questions than answers? Neurosci Res 75:171–183
Hill J, Rom S, Ramirez SH, Persidsky Y (2014) Emerging roles of pericytes in the regulation of the neurovascular unit in health and disease. J Neuroimmune Pharmacol 9:591–605
Abcouwer SF, Lin CM, Shanmugam S et al (2013) Minocycline prevents retinal inflammation and vascular permeability following ischemia-reperfusion injury. J Neuroinflammation 10:149
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
There is no conflict of interest in the article.
Ethical approval
This study was approved by the Ethics Committee of the Affiliated Hospital of Shandong University of Traditional Chinese Medicine, Approval No. (2021) Ethics Audit No. (013)-XY, in accordance with the Declaration of Helsinki guidelines.
Informed consent
Participants provided written consent after receiving detailed information about the study protocol.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article belongs to the topical collection Eye Complications of Diabetes, managed by Giuseppe Querques
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, B., Li, W., Guo, C. et al. Early diagnosis of retinal neurovascular injury in diabetic patients without retinopathy by quantitative analysis of OCT and OCTA. Acta Diabetol 60, 1063–1074 (2023). https://doi.org/10.1007/s00592-023-02086-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-023-02086-z