Abstract
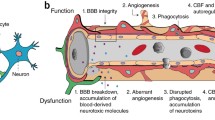
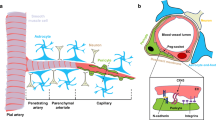
Pericytes of the central nervous system (CNS) are uniquely positioned within a multicellular structure termed the neurovascular unit (NVU) to provide crucial support to blood brain barrier (BBB) formation, maintenance, and stability. Numerous CNS diseases are associated with some aspect of BBB dysfunction. A dysfunction can manifest as one or multiple disruptions to any of the following barriers: physical, metabolic, immunological and transport barrier. A breach in the BBB can notably result in BBB hyper-permeability, endothelial activation and enhanced immune-endothelial interaction. How the BBB is regulated within this integrated unit remains largely unknown, especially as it relates to pericyte-endothelial interaction. We summarize the latest findings on pericyte origin, possible marker expression, and availability within different organ systems. We highlight pericyte-endothelial cell interactions, concentrating on extra- and intra- cellular signaling mechanisms linked to platelet derived growth factor-B, transforming growth factor -β, angiopoietins, Notch, and gap junctions. We discuss the role of pericytes in the NVU under inflammatory insult, focusing on how pericytes may indirectly affect leukocyte CNS infiltration, the direct role of pericyte-mediated basement membrane modifications, and immune responses. We review new findings of pericyte actions in CNS pathologies including Alzheimer’s disease, stroke, multiple sclerosis, diabetic retinopathy, and HIV-1 infection. The uncovering of the regulatory role of pericytes on the BBB will provide key insight into how barrier integrity can be re-established during neuroinflammation.




Similar content being viewed by others
References
Abramsson A, Kurup S, Busse M, Yamada S, Lindblom P, Schallmeiner E, Stenzel D, Sauvaget D, Ledin J, Ringvall M, Landegren U, Kjellen L, Bondjers G, Li JP, Lindahl U, Spillmann D, Betsholtz C, Gerhardt H (2007) Defective N-sulfation of heparan sulfate proteoglycans limits PDGF-BB binding and pericyte recruitment in vascular development. Genes Dev 21:316–331
Allende ML, Proia RL (2002) Sphingosine-1-phosphate receptors and the development of the vascular system. Biochimica Et Biophysica Acta 1582:222–227
Alvarez JI, Cayrol R, Prat A (2011) Disruption of central nervous system barriers in multiple sclerosis. Biochimica Et Biophysica Acta 1812:252–264
Armulik A, Abramsson A, Betsholtz C (2005) Endothelial/pericyte interactions. Circ Res 97:512–523
Armulik A, Mae M, Betsholtz C (2011a) Pericytes and the blood–brain barrier: recent advances and implications for the delivery of CNS therapy. Ther Deliv 2:419–422
Armulik A, Genove G, Betsholtz C (2011b) Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell 21:193–215
Armulik A, Genove G, Mae M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, Johansson BR, Betsholtz C (2010) Pericytes regulate the blood–brain barrier. Nature 468:557–561
Ayres-Sander CE, Lauridsen H, Maier CL, Sava P, Pober JS, Gonzalez AL (2013) Transendothelial migration enables subsequent transmigration of neutrophils through underlying pericytes. PLoS One 8:e60025
Ball HJ, Sanchez-Perez A, Weiser S, Austin CJ, Astelbauer F, Miu J, McQuillan JA, Stocker R, Jermiin LS, Hunt NH (2007) Characterization of an indoleamine 2,3-dioxygenase-like protein found in humans and mice. Gene 396:203–213
Bandopadhyay R, Orte C, Lawrenson JG, Reid AR, De Silva S, Allt G (2001) Contractile proteins in pericytes at the blood–brain and blood-retinal barriers. J Neurocytol 30:35–44
Bell RD, Winkler EA, Sagare AP, Singh I, LaRue B, Deane R, Zlokovic BV (2010) Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron 68:409–427
Beninger RJ, Colton AM, Ingles JL, Jhamandas K, Boegman RJ (1994) Picolinic acid blocks the neurotoxic but not the neuroexcitant properties of quinolinic acid in the rat brain: evidence from turning behaviour and tyrosine hydroxylase immunohistochemistry. Neuroscience 61:603–612
Bonkowski D, Katyshev V, Balabanov RD, Borisov A, Dore-Duffy P (2011) The CNS microvascular pericyte: pericyte-astrocyte crosstalk in the regulation of tissue survival. Fluids and barriers of the CNS 8:8
Brachvogel B, Pausch F, Farlie P, Gaipl U, Etich J, Zhou Z, Cameron T, von der Mark K, Bateman JF, Poschl E (2007) Isolated Anxa5+/Sca-1+ perivascular cells from mouse meningeal vasculature retain their perivascular phenotype in vitro and in vivo. Exp Cell Res 313:2730–2743
Chen Y, Stankovic R, Cullen KM, Meininger V, Garner B, Coggan S, Grant R, Brew BJ, Guillemin GJ (2010) The kynurenine pathway and inflammation in amyotrophic lateral sclerosis. Neurotox Res 18:132–142
Chen YT, Chang FC, Wu CF, Chou YH, Hsu HL, Chiang WC, Shen J, Chen YM, Wu KD, Tsai TJ, Duffield JS, Lin SL (2011) Platelet-derived growth factor receptor signaling activates pericyte-myofibroblast transition in obstructive and post-ischemic kidney fibrosis. Kidney Int 80:1170–1181
Cohen-Salmon M, Maxeiner S, Kruger O, Theis M, Willecke K, Petit C (2004) Expression of the connexin43- and connexin45-encoding genes in the developing and mature mouse inner ear. Cell Tissue Res 316:15–22
da Silva Meirelles L, Caplan AI, Nardi NB (2008) In search of the in vivo identity of mesenchymal stem cells. Stem Cells 26:2287–2299
Dalkara T, Gursoy-Ozdemir Y, Yemisci M (2011) Brain microvascular pericytes in health and disease. Acta Neuropathol 122:1–9
Daneman R, Zhou L, Kebede AA, Barres BA (2010) Pericytes are required for blood–brain barrier integrity during embryogenesis. Nature 468:562–566
Darlington PJ, Boivin MN, Renoux C, Francois M, Galipeau J, Freedman MS, Atkins HL, Cohen JA, Solchaga L, Bar-Or A (2010) Reciprocal Th1 and Th17 regulation by mesenchymal stem cells: Implication for multiple sclerosis. Ann Neurol 68:540–545
DeRuiter MC, Poelmann RE, VanMunsteren JC, Mironov V, Markwald RR, Gittenberger-de Groot AC (1997) Embryonic endothelial cells transdifferentiate into mesenchymal cells expressing smooth muscle actins in vivo and in vitro. Circ Res 80:444–451
Diaz-Flores L, Gutierrez R, Madrid JF, Varela H, Valladares F, Acosta E, Martin-Vasallo P, Diaz-Flores L Jr (2009) Pericytes. Morphofunction, interactions and pathology in a quiescent and activated mesenchymal cell niche. Histol Histopathol 24:909–969
Dimova I, Hlushchuk R, Makanya A, Styp-Rekowska B, Ceausu A, Flueckiger S, Lang S, Semela D, Le Noble F, Chatterjee S, Djonov V (2013) Inhibition of Notch signaling induces extensive intussusceptive neo-angiogenesis by recruitment of mononuclear cells. Angiogenesis 16:921–937
Dohgu S, Banks WA (2013) Brain pericytes increase the lipopolysaccharide-enhanced transcytosis of HIV-1 free virus across the in vitro blood–brain barrier: evidence for cytokine-mediated pericyte-endothelial cell crosstalk. Fluids Barrier CNS 10:23
Dohgu S, Takata F, Yamauchi A, Nakagawa S, Egawa T, Naito M, Tsuruo T, Sawada Y, Niwa M, Kataoka Y (2005) Brain pericytes contribute to the induction and up-regulation of blood–brain barrier functions through transforming growth factor-beta production. Brain Res 1038:208–215
Dore-Duffy P (2008) Pericytes: pluripotent cells of the blood brain barrier. Curr Pharm Des 14:1581–1593
Dore-Duffy P, Cleary K (2011) Morphology and properties of pericytes. Methods Mol Biol 686:49–68
Eberth CJ (1871) Handbuch der Lehre von der Gewegen des Menschen und der Tiere. Leipzig
Enge M, Bjarnegard M, Gerhardt H, Gustafsson E, Kalen M, Asker N, Hammes HP, Shani M, Fassler R, Betsholtz C (2002) Endothelium-specific platelet-derived growth factor-B ablation mimics diabetic retinopathy. EMBO J 21:4307–4316
Engelhardt B, Sorokin L (2009) The blood–brain and the blood-cerebrospinal fluid barriers: function and dysfunction. Semin Immunopathol 31:497–511
Falcon BL, Hashizume H, Koumoutsakos P, Chou J, Bready JV, Coxon A, Oliner JD, McDonald DM (2009) Contrasting actions of selective inhibitors of angiopoietin-1 and angiopoietin-2 on the normalization of tumor blood vessels. Am J Pathol 175:2159–2170
Fallarino F, Grohmann U, Vacca C, Bianchi R, Orabona C, Spreca A, Fioretti MC, Puccetti P (2002) T cell apoptosis by tryptophan catabolism. Cell Death Differ 9:1069–1077
Fang JS, Dai C, Kurjiaka DT, Burt JM, Hirschi KK (2013) Connexin45 regulates endothelial-induced mesenchymal cell differentiation toward a mural cell phenotype. Arterioscler Thromb Vasc Biol 33:362–368
Fiedler U, Reiss Y, Scharpfenecker M, Grunow V, Koidl S, Thurston G, Gale NW, Witzenrath M, Rosseau S, Suttorp N, Sobke A, Herrmann M, Preissner KT, Vajkoczy P, Augustin HG (2006) Angiopoietin-2 sensitizes endothelial cells to TNF-alpha and has a crucial role in the induction of inflammation. Nat Med 12:235–239
Figueroa XF, Duling BR (2009) Gap junctions in the control of vascular function. Antioxid Redox Signal 11:251–266
Gaengel K, Genove G, Armulik A, Betsholtz C (2009) Endothelial-mural cell signaling in vascular development and angiogenesis. Arterioscler Thromb Vasc Biol 29:630–638
Gasque P, Singhrao SK, Neal JW, Wang P, Sayah S, Fontaine M, Morgan BP (1998) The receptor for complement anaphylatoxin C3a is expressed by myeloid cells and nonmyeloid cells in inflamed human central nervous system: analysis in multiple sclerosis and bacterial meningitis. J Immunol 160:3543–3554
Gerhardt H, Betsholtz C (2003) Endothelial-pericyte interactions in angiogenesis. Cell Tissue Res 314:15–23
Goumans MJ, Valdimarsdottir G, Itoh S, Rosendahl A, Sideras P, ten Dijke P (2002) Balancing the activation state of the endothelium via two distinct TGF-beta type I receptors. EMBO J 21:1743–1753
Gu X, Liu XY, Fagan A, Gonzalez-Toledo ME, Zhao LR (2012) Ultrastructural changes in cerebral capillary pericytes in aged Notch3 mutant transgenic mice. Ultrastruct Pathol 36:48–55
Guillemin GJ, Brew BJ (2004) Microglia, macrophages, perivascular macrophages, and pericytes: a review of function and identification. J Leukoc Biol 75:388–397
Guillemin GJ, Smythe G, Takikawa O, Brew BJ (2005) Expression of indoleamine 2,3-dioxygenase and production of quinolinic acid by human microglia, astrocytes, and neurons. Glia 49:15–23
Guillemin GJ, Cullen KM, Lim CK, Smythe GA, Garner B, Kapoor V, Takikawa O, Brew BJ (2007) Characterization of the kynurenine pathway in human neurons. J Neurosci: Official Journal Societ Neurosci 27:12884–12892
Hall CN, Reynell C, Gesslein B, Hamilton NB, Mishra A, Sutherland BA, O’Farrell FM, Buchan AM, Lauritzen M, Attwell D (2014) Capillary pericytes regulate cerebral blood flow in health and disease. Nature 508:55–60
Hamilton NB, Attwell D, Hall CN (2010) Pericyte-mediated regulation of capillary diameter: a component of neurovascular coupling in health and disease. Frontiers in neuroenergetics 2
Hammes HP, Feng Y, Pfister F, Brownlee M (2011) Diabetic retinopathy: targeting vasoregression. Diabetes 60:9–16
Hartung HP, Aktas O, Menge T, Kieseier BC (2014) Immune regulation of multiple sclerosis. Handb Clin Neurol 122:3–14
Hellstrom M, Gerhardt H, Kalen M, Li X, Eriksson U, Wolburg H, Betsholtz C (2001) Lack of pericytes leads to endothelial hyperplasia and abnormal vascular morphogenesis. J Cell Biol 153:543–553
Hirschi KK, Burt JM, Hirschi KD, Dai C (2003) Gap junction communication mediates transforming growth factor-beta activation and endothelial-induced mural cell differentiation. Circ Res 93:429–437
Hosaka K, Yang Y, Seki T, Nakamura M, Andersson P, Rouhi P, Yang X, Jensen L, Lim S, Feng N, Xue Y, Li X, Larsson O, Ohhashi T, Cao Y (2013) Tumour PDGF-BB expression levels determine dual effects of anti-PDGF drugs on vascular remodelling and metastasis. Nat Commun 4:2129
Hu B, Hissong BD, Carlin JM (1995) Interleukin-1 enhances indoleamine 2,3-dioxygenase activity by increasing specific mRNA expression in human mononuclear phagocytes. J Interferon & Cytokine Res : Official J Int Societ Interferon and Cytokine Res 15:617–624
Hughes S, Chan-Ling T (2004) Characterization of smooth muscle cell and pericyte differentiation in the rat retina in vivo. Invest Ophthalmol Vis Sci 45:2795–2806
Jaeger LB, Dohgu S, Sultana R, Lynch JL, Owen JB, Erickson MA, Shah GN, Price TO, Fleegal-Demotta MA, Butterfield DA, Banks WA (2009) Lipopolysaccharide alters the blood–brain barrier transport of amyloid beta protein: a mechanism for inflammation in the progression of Alzheimer’s disease. Brain Behav Immun 23:507–517
Jeansson M, Gawlik A, Anderson G, Li C, Kerjaschki D, Henkelman M, Quaggin SE (2011) Angiopoietin-1 is essential in mouse vasculature during development and in response to injury. J Clin Invest 121:2278–2289
Jin S, Hansson EM, Tikka S, Lanner F, Sahlgren C, Farnebo F, Baumann M, Kalimo H, Lendahl U (2008) Notch signaling regulates platelet-derived growth factor receptor-beta expression in vascular smooth muscle cells. Circ Res 102:1483–1491
Kirk J, Plumb J, Mirakhur M, McQuaid S (2003) Tight junctional abnormality in multiple sclerosis white matter affects all calibres of vessel and is associated with blood–brain barrier leakage and active demyelination. J Pathol 201:319–327
Krueger M, Bechmann I (2010) CNS pericytes: concepts, misconceptions, and a way out. Glia 58:1–10
Kruger O, Plum A, Kim JS, Winterhager E, Maxeiner S, Hallas G, Kirchhoff S, Traub O, Lamers WH, Willecke K (2000) Defective vascular development in connexin 45-deficient mice. Development 127:4179–4193
Kume T (2012) Ligand-dependent Notch signaling in vascular formation. Adv Exp Med Biol 727:210–222
Kusao I, Shiramizu B, Liang CY, Grove J, Agsalda M, Troelstrup D, Velasco VN, Marshall A, Whitenack N, Shikuma C, Valcour V (2012) Cognitive performance related to HIV-1-infected monocytes. J Neuropsychiat Clinic Neurosci 24:71–80
Kwidzinski E, Bechmann I (2007) IDO expression in the brain: a double-edged sword. J Mol Med 85:1351–1359
Lamalice L, Le Boeuf F, Huot J (2007) Endothelial cell migration during angiogenesis. Circ Res 100:782–794
Lee GK, Park HJ, Macleod M, Chandler P, Munn DH, Mellor AL (2002) Tryptophan deprivation sensitizes activated T cells to apoptosis prior to cell division. Immunology 107:452–460
Li F, Lan Y, Wang Y, Wang J, Yang G, Meng F, Han H, Meng A, Wang Y, Yang X (2011) Endothelial Smad4 maintains cerebrovascular integrity by activating N-cadherin through cooperation with Notch. Dev Cell 20:291–302
Lindahl P, Johansson BR, Leveen P, Betsholtz C (1997) Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science 277:242–245
Liu H, Zhang W, Kennard S, Caldwell RB, Lilly B (2010) Notch3 is critical for proper angiogenesis and mural cell investment. Circ Res 107:860–870
Liu Y, Wada R, Yamashita T, Mi Y, Deng CX, Hobson JP, Rosenfeldt HM, Nava VE, Chae SS, Lee MJ, Liu CH, Hla T, Spiegel S, Proia RL (2000) Edg-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. J Clin Invest 106:951–961
Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, Daly TJ, Davis S, Sato TN, Yancopoulos GD (1997) Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science 277:55–60
McCullough PA, Olobatoke A, Vanhecke TE (2011) Galectin-3: a novel blood test for the evaluation and management of patients with heart failure. Rev Cardiovasc Med 12:200–210
Munn DH, Shafizadeh E, Attwood JT, Bondarev I, Pashine A, Mellor AL (1999) Inhibition of T cell proliferation by macrophage tryptophan catabolism. J Experiment Med 189:1363–1372
Nakagawa S, Castro V, Toborek M (2012) Infection of human pericytes by HIV-1 disrupts the integrity of the blood–brain barrier. J Cell Mol Med 16:2950–2957
Nees S, Weiss DR, Senftl A, Knott M, Forch S, Schnurr M, Weyrich P, Juchem G (2012) Isolation, bulk cultivation, and characterization of coronary microvascular pericytes: the second most frequent myocardial cell type in vitro. Am J Physiol Heart Circulat Physiol 302:H69–84
Nishioku T, Matsumoto J, Dohgu S, Sumi N, Miyao K, Takata F, Shuto H, Yamauchi A, Kataoka Y (2010) Tumor necrosis factor-alpha mediates the blood–brain barrier dysfunction induced by activated microglia in mouse brain microvascular endothelial cells. J Pharmacol Sci 112:251–254
Noda K, Nakao S, Zandi S, Sun D, Hayes KC, Hafezi-Moghadam A (2014) Retinopathy in a novel model of metabolic syndrome and type 2 diabetes: new insight on the inflammatory paradigm. FASEB journal : official publication of the Federation of American Societies for Experimental Biology
Orlidge A, D’Amore PA (1987) Inhibition of capillary endothelial cell growth by pericytes and smooth muscle cells. J Cell Biol 105:1455–1462
Owe-Young R, Webster NL, Mukhtar M, Pomerantz RJ, Smythe G, Walker D, Armati PJ, Crowe SM, Brew BJ (2008) Kynurenine pathway metabolism in human blood–brain-barrier cells: implications for immune tolerance and neurotoxicity. J Neurochem 105:1346–1357
Ozen I, Boix J, Paul G (2012) Perivascular mesenchymal stem cells in the adult human brain: a future target for neuroregeneration? Clinic Translat Med 1:30
Paik JH, Skoura A, Chae SS, Cowan AE, Han DK, Proia RL, Hla T (2004) Sphingosine 1-phosphate receptor regulation of N-cadherin mediates vascular stabilization. Genes Dev 18:2392–2403
Patan S (1998) TIE1 and TIE2 receptor tyrosine kinases inversely regulate embryonic angiogenesis by the mechanism of intussusceptive microvascular growth. Microvasc Res 56:1–21
Persidsky Y, Ramirez SH, Haorah J, Kanmogne GD (2006) Blood–brain barrier: structural components and function under physiologic and pathologic conditions. J Neuroimmun Pharmacol :Official J SocietNeuroImmun Pharmacol 1:223–236
Peters A, Sethares C (2012) Age-related changes in the morphology of cerebral capillaries do not correlate with cognitive decline. J Comp Neurol 520:1339–1347
Pfister F, Wang Y, Schreiter K, vom Hagen F, Altvater K, Hoffmann S, Deutsch U, Hammes HP, Feng Y (2010) Retinal overexpression of angiopoietin-2 mimics diabetic retinopathy and enhances vascular damages in hyperglycemia. Acta Diabetol 47:59–64
Pieper C, Marek JJ, Unterberg M, Schwerdtle T, Galla HJ (2014) Brain capillary pericytes contribute to the immune defense in response to cytokines or LPS in vitro. Brain Res 1550:1–8
Pillai DR, Dittmar MS, Baldaranov D, Heidemann RM, Henning EC, Schuierer G, Bogdahn U, Schlachetzki F (2009) Cerebral ischemia-reperfusion injury in rats–a 3 T MRI study on biphasic blood–brain barrier opening and the dynamics of edema formation. J Cerebral Blood Flow Metabol: Official J Int Societ Cerebral Blood Flow Metabol 29:1846–1855
Piquer-Gil M, Garcia-Verdugo JM, Zipancic I, Sanchez MJ, Alvarez-Dolado M (2009) Cell fusion contributes to pericyte formation after stroke. J Cerebral Blood Flow Metabol: Official J Int Societ Cerebral Blood Flow Metabol 29:480–485
Potula R, Poluektova L, Knipe B, Chrastil J, Heilman D, Dou H, Takikawa O, Munn DH, Gendelman HE, Persidsky Y (2005) Inhibition of indoleamine 2,3-dioxygenase (IDO) enhances elimination of virus-infected macrophages in an animal model of HIV-1 encephalitis. Blood 106:2382–2390
Proebstl D, Voisin MB, Woodfin A, Whiteford J, D’Acquisto F, Jones GE, Rowe D, Nourshargh S (2012) Pericytes support neutrophil subendothelial cell crawling and breaching of venular walls in vivo. J Experiment Med 209:1219–1234
Pun PB, Lu J, Moochhala S (2009) Involvement of ROS in BBB dysfunction. Free Radic Res 43:348–364
Rodriguez A, Friman T, Kowanetz M, van Wieringen T, Gustafsson R, Sundberg C (2013) Phenotypical differences in connective tissue cells emerging from microvascular pericytes in response to overexpression of PDGF-B and TGF-beta1 in normal skin in vivo. Am J Pathol 182:2132–2146
Rouget C (1873) Memoire sur le developpement, la structures et les proprietes des capillaires sanguins et lymphatiques. Archs Physiol Norm Pathol 5:603–633
Rowe RG, Weiss SJ (2008) Breaching the basement membrane: who, when and how? Trends Cell Biol 18:560–574
Sa-Pereira I, Brites D, Brito MA (2012) Neurovascular unit: a focus on pericytes. Mol Neurobiol 45:327–347
Sagare AP, Bell RD, Zhao Z, Ma Q, Winkler EA, Ramanathan A, Zlokovic BV (2013) Pericyte loss influences Alzheimer-like neurodegeneration in mice. Nat Commun 4:2932
Schlondorff D, Banas B (2009) The mesangial cell revisited: no cell is an island. J Am Societ Nephrol : JASN 20:1179–1187
Schrimpf C, Xin C, Campanholle G, Gill SE, Stallcup W, Lin SL, Davis GE, Gharib SA, Humphreys BD, Duffield JS (2012) Pericyte TIMP3 and ADAMTS1 modulate vascular stability after kidney injury. J Am Societ Nephrol : JASN 23:868–883
Sengillo JD, Winkler EA, Walker CT, Sullivan JS, Johnson M, Zlokovic BV (2013) Deficiency in mural vascular cells coincides with blood–brain barrier disruption in Alzheimer’s disease. Brain Pathol 23:303–310
Smith DG, Guillemin GJ, Pemberton L, Kerr S, Nath A, Smythe GA, Brew BJ (2001) Quinolinic acid is produced by macrophages stimulated by platelet activating factor, Nef and Tat. J Neurovirol 7:56–60
Smith SW, Eardley KS, Croft AP, Nwosu J, Howie AJ, Cockwell P, Isacke CM, Buckley CD, Savage CO (2011) CD248+ stromal cells are associated with progressive chronic kidney disease. Kidney Int 80:199–207
Soriano P (1994) Abnormal kidney development and hematological disorders in PDGF beta-receptor mutant mice. Genes Dev 8:1888–1896
Stefanska A, Peault B, Mullins JJ (2013) Renal pericytes: multifunctional cells of the kidneys. Arch Eur J Physiol 465:767–773
Tachibana K, Jones N, Dumont DJ, Puri MC, Bernstein A (2005) Selective role of a distinct tyrosine residue on Tie2 in heart development and early hematopoiesis. Mol Cell Biol 25:4693–4702
Takikawa O, Kuroiwa T, Yamazaki F, Kido R (1988) Mechanism of interferon-gamma action. Characterization of indoleamine 2,3-dioxygenase in cultured human cells induced by interferon-gamma and evaluation of the enzyme-mediated tryptophan degradation in its anticellular activity. J BiolChem 263:2041–2048
Takikawa O, Tagawa Y, Iwakura Y, Yoshida R, Truscott RJ (1999) Interferon-gamma-dependent/independent expression of indoleamine 2,3-dioxygenase. Studies with interferon-gamma-knockout mice. Adv Exp Med Biol 467:553–557
Tallquist MD, French WJ, Soriano P (2003) Additive effects of PDGF receptor beta signaling pathways in vascular smooth muscle cell development. PLoS Biol 1:E52
Van Geest RJ, Klaassen I, Vogels IM, Van Noorden CJ, Schlingemann RO (2010) Differential TGF-{beta} signaling in retinal vascular cells: a role in diabetic retinopathy? Invest Ophthalmol Vis Sci 51:1857–1865
von Tell D, Armulik A, Betsholtz C (2006) Pericytes and vascular stability. Exp Cell Res 312:623–629
Wang S, Cao C, Chen Z, Bankaitis V, Tzima E, Sheibani N, Burridge K (2012) Pericytes regulate vascular basement membrane remodeling and govern neutrophil extravasation during inflammation. PloS one 7:e45499
Wang S, Voisin MB, Larbi KY, Dangerfield J, Scheiermann C, Tran M, Maxwell PH, Sorokin L, Nourshargh S (2006) Venular basement membranes contain specific matrix protein low expression regions that act as exit points for emigrating neutrophils. J Experiment Med 203:1519–1532
Wang Y, Pan L, Moens CB, Appel B (2014) Notch3 establishes brain vascular integrity by regulating pericyte number. Development 141:307–317
Winkler EA, Bell RD, Zlokovic BV (2011a) Central nervous system pericytes in health and disease. Nat Neurosci 14:1398–1405
Winkler EA, Bell RD, Zlokovic BV (2011b) Lack of Smad or Notch leads to a fatal game of brain pericyte hopscotch. Dev Cell 20:279–280
Wohlfart P, Lin J, Dietrich N, Kannt A, Elvert R, Herling AW, Hammes HP (2014) Expression patterning reveals retinal inflammation as a minor factor in experimental retinopathy of ZDF rats. Acta diabetologica
Yadav R, Larbi KY, Young RE, Nourshargh S (2003) Migration of leukocytes through the vessel wall and beyond. Thromb Haemost 90:598–606
Yemisci M, Gursoy-Ozdemir Y, Vural A, Can A, Topalkara K, Dalkara T (2009) Pericyte contraction induced by oxidative-nitrative stress impairs capillary reflow despite successful opening of an occluded cerebral artery. Nat Med 15:1031–1037
Zimmerman KW (1923) Der feinere bau der blutcapillares. Z Anat Entwicklungsgesch 68:3–109
Zlokovic BV (2008) The blood–brain barrier in health and chronic neurodegenerative disorders. Neuron 57:178–201
Zlokovic BV (2011) Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci 12:723–738
Acknowledgments
This work was supported by National Institutes of Health (NIH) grants NS086570 (SHR), MH65151 and AA015913 (YP), DA013429 (SHR, YP), and The Shriners Hospital for Children grant 85110-PHI-14 (SHR).
The authors express their grateful acknowledgement for proofreading and editing to Nancy L. Reichenbach.
Conflict of Interest
The authors of this manuscript declare that there are no actual or potential conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hill, J., Rom, S., Ramirez, S.H. et al. Emerging Roles of Pericytes in the Regulation of the Neurovascular Unit in Health and Disease. J Neuroimmune Pharmacol 9, 591–605 (2014). https://doi.org/10.1007/s11481-014-9557-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11481-014-9557-x