Abstract
Purpose
In this longitudinal cohort study, we investigated the relationship of disc degeneration (DD) after pubertal growth spurt to future low back pain (LBP).
Methods
A group of healthy volunteers underwent a semi-structured interview about LBP without trauma and a 1.5T lumbar MRI at ages 18 and 34. A Pfirrmann Summary Score (PSS) was calculated by adding up the Pfirrmann grades of the three lowest lumbar discs of each subject (range 3–15). The relationship of PSS at age 18 to LBP at age 34 was analyzed.
Results
Forty-one participants had full data at both time points. Mean PSS at age 18 was 6.8 (SD 1.1) and 5.6 (SD 1.2) for participants with or without LBP at age 34, respectively (p = 0.009). The OR (95% CI) of PSS at age 18 for LBP at age 34 was 5.46 (1.22 to 24.47) when adjusted for sex, BMI, smoking and physical activity. All participants but one with PSS greater than 6 at age 18 reported LBP at age 34.
Conclusion
This is the first study to suggest that DD may be associated with future LBP and the critical time frame seems to be the pubertal growth spurt. Every 1-point increase in Pfirrmann grade at age 18 increased the risk of LBP 5.5-fold at age 34 when adjusted for sex, BMI, smoking and physical activity at age 34. All participants but one with at least one disc with Pfirrmann grade 3 or higher at age 18 reported LBP at age 34.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite a wealth of research on the relationship between the clinical symptom of low back pain (LBP) and degenerative findings on magnetic resonance imaging (MRI), the etiology of LBP remains poorly understood. Especially disc degeneration (DD) has been studied extensively in relation to LBP, albeit with conflicting results. While MRI findings traditionally associated with LBP are frequently observed in asymptomatic individuals [1], DD appears to be more prevalent among both adolescents [2] and adults [3] experiencing LBP. In the latest observational studies, the prevalence of DD has been higher in individuals with LBP compared to asymptomatic populations [4,5,6]. Specifically, Smith et al. reported a significantly higher risk of consistent LBP at the age of 27 years in individuals with DD graded as Pfirrmann 3 or higher, particularly if the disc changes were multilevel [4]. Additionally, both Tertimo et al. and Jamaludin et al. identified severe DD, defined as at least one disc with Pfirrmann grade 5 or a higher lumbar DD sum score [5], or Pfirrmann grade 4 and 5 [6], increasing the likelihood of experiencing LBP among individuals under 50 years of age.
Few studies have investigated whether DD on MRI predicts future LBP. A systematic review did not establish an association between baseline MRI findings and future LBP, at least partly due to differences in patient populations, MRI findings of interest and clinical outcomes [7]. Moreover, baseline MRI findings and the rate of DD progression do not seem to be associated with the risk of future LBP in a 5- to 30-year follow-ups [8,9,10,11]. Some evidence, however, suggests that baseline DD may be associated with increased LBP severity at a 6.4-year follow-up [9].
In previous cross-sectional studies, the occurrence of DD, defined as Pfirrmann grade 3 or higher, has been rare among asymptomatic pediatric populations aged under 10 [12, 13]. However, DD appears to become more prevalent during the pubertal growth spurt [14]. Consequently, early adulthood emerges as a relevant period to investigate the relationship between DD and subsequent LBP. In this longitudinal cohort study involving a group of healthy volunteers, our goal was first, to analyze the relationship between lumbar DD at the age of 18 and future LBP at the age of 34, and second, to investigate whether a specific extent of DD after the pubertal growth spurt increases the likelihood of LBP in adulthood.
Methods
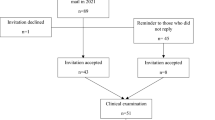
The data of the present study were analyzed from participants enrolled in a longitudinal prospective study on the development of lumbar intervertebral discs from childhood to adulthood. In 1994, 208 2nd graders were randomly selected from the urban capital area of Helsinki and invited to participate in the study. Of the 108 children interested in participating, 94 underwent the baseline examination at the age of 8–9 years. Follow-up examinations were conducted at the ages of 11–12 years (n = 81) and 18–19 years (n = 71). In 2021, all those participants whose contact details were known (n = 89 of the original 94 participants) were invited to participate in a long-term follow-up at the age of 34 (n = 48). Hereby, we concentrate on the time points of 18 and 34 years of age and report the results of the participants who had a full data set at these study time points (n = 41).
All study time points included a semi-structured interview, a standard clinical examination, and a lumbar spine MRI. At the age of 34, the following patient-reported outcome measures (PROM) were added: NRS, Oswestry Disability Index (ODI), EQ-5D-5L and IPAQ (International Physical Activity Questionnaire). The physical activity level of the participants was reported in metabolic equivalent (MET) hours per month.
Semi-structured interview and clinical examination
The semi-structured interview inquired about a history of LBP without specific trauma (last week/last month/last year/earlier), sports activities (discipline, for how long and how many hours per week), and smoking status (never, quitted, currently smoking).
The participants’ height and sitting height were measured with a stadiometer and weight with a balance-beam scale. Body Mass Index (BMI) was calculated using the standard formula for adults (weight in kilograms divided by the square of height in meters).
MRI investigation
All the MRI investigations were performed before 10 am to prevent possible diurnal variation in the disc signal intensity (SI). The MRIs at the ages of 18 and 34 were acquired using a 1.5T high-field scanner with a dedicated spine coil (at age 18: Siemens Symphony, Siemens, Erlangen, Germany; at age 34: Canon Vantage Orian, Canon Medical Systems Corporation, Otawara, Japan).
According to the original study protocol, at the age of 18 only T2 sagittal sequences were performed using the following parameters: TR 4630 ms, TE 107ms, FOV 280, image matrix 384 × 288, slice thickness 4.0 mm, interslice gap 0.4 mm, acq.2.
At the age of 34 the imaging parameters for a standard lumbar MRI were: T2 sagittal sequence (TR 4950ms, TE 120 ms, FOV 300, slice thickness 3.5 mm, interslice gap 0.3 mm, acq2); T1 sagittal sequence (TR 500ms, TE 9 ms, FOV 300, slice thickness 3.5 mm, interslice gap 0.3 mm, acq1); STIR sagittal sequence (TR 4600ms, TE 50 ms, TI 125, FOV 300, slice thickness 3.5 mm, interslice gap 0.3 mm, acq1); T2 axial sequence with imaging planes in the direction of the three lowest discs 1 + 1 + 1 (TR 3150ms, TE 120 ms, FOV 220, slice thickness 3 mm, interslice gap 0.3 mm, acq1).
MRI assessment
The three lowest lumbar discs were visually assessed on mid-sagittal T2-weighted images using the Pfirrmann classification [15]. A musculoskeletal radiologist (fourth author) and a spine surgeon (last author) independently classified the intervertebral discs without knowledge of the participants LBP status or previous DD findings. In case of discrepancy, the independent assessment of a third evaluator (fifth author) was used for consensus. The individual Pfirrmann grades of the three discs were added up for a Pfirrmann Summary Score (PSS, range 3–15) [16].
This study was conducted according to the Declaration of Helsinki for research on human participants. The ethical approval for the original study was granted by the Ethics Committee of the Invalid Foundation (later Research Institute Orton) on January 22, 1993. For the long-term follow-up, the study protocol was approved by the Ethics Committee of Helsinki University Hospital on December 9, 2020. This approval authorized us to gain access to the contact details of the original study participants from the Digital and Population Data Services Agency, using their individual social security numbers. Furthermore, a written informed consent was obtained from the parents of each study participant before commencement of the study and from the participants themselves before the long-term follow-up.
When applicable, this manuscript follows the STROBE guidelines for reporting observational studies.
Statistical analysis
Summary statistics were described using mean and standard deviation (SD), median and interquartile range (IQR), or numbers as percentages. Statistical evaluations between groups were analyzed using Student’s t-test, Mann–Whitney U test, Pearson’s chi-squared test, and Fisher’s exact test. Longitudinal data on the PSS was analyzed between groups with multilevel mixed-effects linear regression models using unstructured covariance structure. Mixed-effects models included main effects (group and time) and their interaction. Exact logistic regression models were used to investigate factors related to LBP. Stata 17.0, StataCorp LP (College Station, TX, USA) statistical package was used for the analysis.
Results
Thirty-one (31) participants (76%) reported LBP at the age of 34 years. A statistically significant difference in the use of pain medication (0% vs. 35%, p = 0.035) and in ODI (22.9 vs. 25.2, p = 0.045) was noticed among the participants without and with LBP at the age of 34, respectively. Table 1 for characteristics of the study population.
PSS at the age of 18 was significantly higher among participants with LBP at the age of 34 compared to participants without LBP at the age of 34: 6.8 (95% Confidence Interval [CI] 6.4–7.2) versus 5.6 (95% CI 4.7–6.2), respectively (p = 0.009), Fig. 1. At the age of 34, the PSS was 7.6 (95% CI 7.1–8.2) and 6.9 (95% CI 6.4–7.6) among participants with and without LBP, respectively (p = 0.20), Fig. 1.
The OR (95% CI) of PSS at the age of 18 for LBP at the age of 34 was 3.5 (1.08 to 11.8) when adjusted for sex and 5.46 (1.22 to 24.47) when adjusted for sex, BMI, smoking and level of physical activity [17]. The OR (95% CI) of PSS at the age of 34 for actual LBP at the age of 34 was 1.61 (0.76 to 3.42).
All participants except one with PSS higher than 6 at the age of 18 reported LBP at the age of 34.
Discussion
In the current study, we utilized data from a longitudinal prospective study on the development of lumbar intervertebral discs from childhood to adulthood in a cohort of healthy randomly selected volunteers. Our specific aim was to investigate the relationship between DD after the pubertal growth spurt and future LBP. Further, we wanted to examine whether a specific extent of DD in early adulthood is associated with LBP later in life. The main findings of our study were two-fold: every 1-point increase in the Pfirrmann grade of one of the three lowest discs at the age of 18 increased the risk of LBP 5.5-fold at the age of 34 when adjusted with factors associated with occurrence of LBP [17]. Further, having at least one disc with a Pfirrmann grade higher than 2 at the age of 18 appeared to increase the likelihood of LBP at the age of 34.
Few longitudinal studies have explored the relationship between baseline MRI findings and future LBP. In a systematic review, no consistent relationship between baseline MRI findings and a clinically important future LBP was identified [7]. More recent longitudinal cohort studies have not found a repationship between baseline MRI findings and future LBP in a 5-year [8], 6.4-year [9], 10-year [10] or 30-year [11] follow-up. It is pertinent to note that in these prior studies, the study populations were older compared to the current study. In the systematic review, in studies focusing on DD as the MRI finding of interest [7], the mean age of the participants at baseline ranged from 35.2 to 44 years. In the longitudinal study conducted by Iordanova Schistad et al. [8], the mean age of participants at baseline was 47 years for men and 46 years for women. While Kasch et al. [9] included younger subjects aged between 20 and 40 years, the mean age of the study population at baseline was 53.0 years (SD 13.7). Additionally, they did not report the follow-up results based on age categories. Finally, the study population of Tonosu et al. [10] had an average age of 44.9 years at the 10-year follow-up time point.
Sääksjärvi et al. [11] conducted a 30-year MRI follow-up on 20-year-old males, which renders their study comparable to the present one. They concluded that early lumbar DD at a disc level was associated with accelerated DD at that specific disc level but not with future LBP. However, it is worth noting that all their study participants had baseline LBP severe enough to discharge them from military service. Moreover, the authors did not have access to the baseline MRI studies for assessing morphological disc changes and thus had to rely on existing relative values of disc SI for comparison. Their method involved using the disc with the highest SI as a reference in a computerized SI measurement, which could introduce error if the disc considered the “healthiest” is already degenerated. Consequently, due to these methodological disparities, comparing our findings with those of Sääksjärvi et al. is difficult. In another prospective MRI investigation of 20-22-year-old females, almost one third of the subjects had DD at baseline; in these subjects, the DD progressed rapidly during the 10-year follow-up [18]. However, the authors did not investigate the relationship between baseline DD and future LBP.
In our study, a significant difference in the extent of DD between participants with or without LBP at the age of 34 was already observed at the age of 18. Notably, by the age of 34, the degree of DD in asymptomatic participants had progressed to a level comparable to their symptomatic peers. This suggests that the relevant time frame for understanding the relationship between DD and future LBP may be earlier than previously studied, i.e., after the pubertal growth spurt. Comparing our results with previous literature suggests that establishing a relationship between baseline MRI findings and future LBP in older age groups may pose challenges.
To our knowledge, this is the first study to investigate whether a specific extent of DD at baseline increases the likelihood of future LBP. Notably, all participants but one with a PSS above 6 at the age of 18 reported LBP at the age of 34. Since Pfirrmann grade 1 was rare at the age of 18 (12% of all analyzed discs), Pfirrmann grade 2 seems to represent a “normal” disc morphology in an 18-year-old individual. Consequently, any disc with a Pfirrmann grade 3 or higher at this age may indicate DD and increase the likelihood of LBP in adulthood. In our asymptomatic participants, age-related degenerative changes of Pfirrmann grade 3 or higher did not manifest until the third and fourth decades of life.
Our study has several strengths. To our knowledge this is the first study to investigate the development of lumbar discs from childhood to adulthood. Due to the prospective longitudinal study design the disc changes reported herein represent true age-associated findings. The study participants comprise a group of randomly chosen volunteers drawn from a general population. Further, at no point during the study were the participants aware of their MRI findings. During the semi-structured interview, the questions about LBP were presented as only one of several topics.
Some limitations need to be considered when interpreting our results. Selection bias may impact the generalizability of our findings. Families who initially opted to enroll their children in the study might have a stronger family history of LBP compared to those who did not participate. Additionally, participants who took part in the long-term follow-up may differ from those who did not respond to our invitation, specifically in terms of occurrence and intensity of LBP. Furthermore, with self-reported LBP, particularly concerning life-time prevalence, potential recall bias should be acknowledged. Participants may have forgotten about earlier episodes of LBP, albeit less likely more severe or recurrent episodes. However, the cumulative life-time prevalence of LBP in our study population (76%) corresponds to a previous report on a larger cohort in the same age group [19]. Longitudinal studies encounter problems of missing data due to multiple data collection points and participant attrition [20]. In the present analysis, we only included participants with complete data at the ages of 18 and 34. Further, our analysis focused solely on DD whereas other morphological changes, either individually or collectively, may be associated with LBP. Indeed, clusters of degenerative findings have been suggested to be more predictive of clinical outcome [7]. A recent narrative review identified type I Modic changes, DD, endplate defects, disc herniation, spinal canal stenosis, nerve compression, and muscle fat infiltration as having the highest probability of being linked to LBP [21]. On the other hand, in a cross-sectional study of a birth cohort comprising 1303 middle-aged adults, a significant association between DD and LBP was observed, independent of other pain-related imaging findings [5]. In our study population, no statistically significant difference at the age of 34 was found between participants with or without LBP regarding the occurrence of Modic changes, high-intensity zones (HIZ) or disc protrusions (data not shown). While our study population size might have been underpowered to detect associations between these changes and LBP, a statistically significant association of PSS after pubertal growth spurt with LBP in adulthood was established.
It is important to bear in mind that we did not find any relationship between the degree of DD and current LBP in our study population. Thus, we do not suggest that young adults with more widespread DD after the pubertal growth spurt should be labeled as having a “degenerative disc disease” and a future of chronic disabling LBP. We believe, however, that our results may have clinical relevance to those young adults who are incidentally “diagnosed” as having a more widespread DD. A randomized clinical trial has shown that this age group is receptive to health-promoting lifestyle modifications [22]. Specifically for LBP, people who adopt lifestyle behaviors such as adequate levels of physical activity, optimal sleep quality, ideal BMI and non-smoking status, are likely to cope better with their LBP [23].
Conclusions
Our findings suggest that disc changes indicative of future LBP may emerge during the pubertal growth spurt. Specifically, each 1-point increase in the Pfirrmann grade in one of the three lowest lumbar discs at the age of 18 resulted in a 5.5-fold increase in the risk of LBP at the age of 34 when adjusted for factors associated with LBP (sex, BMI, smoking and physical activity level). The presence of lumbar discs with a Pfirrmann grade higher than 2 after the pubertal growth spurt may increase the likelihood of experiencing LBP in adulthood. Future studies should aim to elucidate the complex interplay between DD and LBP across various age groups.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Brinjikji W, Luetmer PH, Comstock B, Bresnahan BW, Chen LE, Deyo RA, Halabi S, Turner JA, Avins AL, James K, Wald JT, Kallmes DF, Jarvik JG (2015) Systematic literature review of imaging features of spinal degeneration in asymptomatic populations. Am J Neuroradiol 36:811–816. https://doi.org/10.3174/ajnr.A4173
van den Heuvel MM, Oei EHG, Bierma-Zeinstra SMA, van Middelkoop M (2020) The prevalence of abnormalities in the pediatric spine on MRI. A systematic review and meta-analysis. Spine 45:E1185–E1196. https://doi.org/10.1097/BRS.0000000000003527
Brinjikji W, Diehn FE, Jarvik JG, Carr CM, Kallmes DF, Murad MH, Luetmer PH (2015) MRI findings of disc degeneration are more prevalent in adults with low back pain than in asymptomatic controls: a systematic review and meta-analysis. Am J Neuroradiol 36:2394–2399. https://doi.org/10.3174/ajnr.A4498
Smith A, Hancock M, O’Hanlon S, Krieser M, O’Sullivan P, Cicuttini F, Straker L, Adler B, Wang YY, Karppinen J, Samartzis D, Beales D, Coenen P, Kent P (2021) The association between different trajectories of low back pain and degenerative imaging findings in young adult participants within the Raine Study. Spine 47:269–276. https://doi.org/10.1097/BRS.0000000000004171
Mertimo T, Karppinen J, Niinimäki J, Blanco R, Määttä J, Kankaanpää M, Oura P (2022) Association of lumbar disc degeneration with low back pain in middle age in the Northern Finland Birth Cohort 1966. BMC Musculoskelet Disord 23:359. https://doi.org/10.1186/s12891-022-05302-z
Jamaludin A, Kadir T, Zisserman A, McCall I, Williams FMK, Lang H, Buchanan E, Urban JPG, Fairbank JCT (2023) ISSLS PRIZE in Clinical Science 2023: comparison of degenerative MRI features of the intervertebral disc between those with and without chronic low back pain. An exploratory study of two large female populations using automated annotation. Eur Spine J 32:1504–1516. https://doi.org/10.1007/s00586-023-07604-9
Steffens D, Hancock MJ, Maher CG, Williams C, Jensen TS, Latimer J (2014) Does magnetic resonance imaging predict future low back pain? A systematic review. Eur J Pain 18:755–765. https://doi.org/10.1002/j.1532-2149.2013.00427.x
Iordanova Schistad E, Bjorland S, Røe C, Gjerstad J, Vetti N, Myhre K, Espeland A (2019) Five-year development of lumbar disc degeneration – a prospective study. Skeletal Radiol 48:871–879. https://doi.org/10.1007/s00256-018-3062-x
Kasch R, Truthmann J, Hancock MJ, Maher CG, Otto M, Nell C, Reichwein N, Bülow R, Chenot J-F, Hofer A, Wassilew G, Schmidt CO (2022) Association of lumbar MRI findings with current and future back pain in a population-based cohort study. Spine 47:201–211. https://doi.org/10.1097/BRS.0000000000004198
Tonosu J, Oka H, Higashikawa A, Okazaki H, Tanaka S, Matsudaira K (2017) The associations between magnetic resonance imaging findings and low back pain: a 10-year longitudinal analysis. PLoS ONE 12:e0188057. https://doi.org/10.1371/journal.pone.0188057
Sääksjärvi S, Kerttula L, Luoma K, Paajanen H, Waris E (2020) Disc degeneration of young low back pain patients. A prospective 30-year follow-up MRI study. Spine 45:1341–1347. https://doi.org/10.1097/BRS.0000000000003548
Urrutia J, Zamora T, Prada C (2016) The prevalence of degenerative or incidental findings in the lumbar spine of pediatric patients: a study using magnetic resonance imaging as a screening tool. Eur Spine J 25:596–601. https://doi.org/10.1007/s00586-015-4099-3
van den Heuvel MM, Oei EHG, Renkens JJM, Bierma-Zeinstra SMA, van Middelkoop M (2021) Structural spinal abnormalities on MRI and associations with weight status in a general pediatric population. Spine J 21:465–476. https://doi.org/10.1016/j.spinee.2020.10.003
Takatalo J, Karppinen J, Niinimäki J, Taimela S, Näyhä S, Järvelin M-R, Kyllönen E, Tervonen O (2009) Prevalence of degenerative imaging findings in lumbar magnetic resonance imaging among young adults. Spine 34:1716–1721. https://doi.org/10.1097/BRS.0b013e3181ac5fec
Pfirrmann CWA, Metzdorf A, Zanetti M, Hodler J, Boos N (2001) Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine 26:1873–1878. https://doi.org/10.1097/00007632-200109010-00011
Määttä JH, Karppinen JI, Luk KDK, Cheung KMC, Samartzis D (2015) Phenotype profiling of Modic changes of the lumbar spine and its association with other MRI phenotypes: a large-scale population-based study. Spine J 15:1933–1942. https://doi.org/10.1016/j.spinee.2015.06.056
Hartvigsen J, Hancock MJ, Kongsted A, Louw Q, Ferreira ML, Genevay S, Hoy D, Karppinen J, Pransky G, Sieper J, Smeets RJ, Underwood M (2018) What low back pain is and why we need to pay attention. Lancet 391:2356–2367. https://doi.org/10.1016/S0140-6736(18)30480-X
Makino H, Kawaguchi Y, Seki S, Nakano M, Yasuda T, Suzuki K, Ikegawa S, Kimura T (2017) Lumbar disc degeneration progression in young women in their 20’s: a prospective ten-year follow up. J Orthop Sci 22:635–640. https://doi.org/10.1016/j.jos.2017.03.015
Harreby M, Kjer J, Hesselsøe G, Neergaard K (1996) Epidemiological aspects and risk factors for low back pain in 38-year-old men and women: a 25-year prospective cohort study of 640 school children. Eur Spine J 5:312–318. https://doi.org/10.1007/BF00304346
Powney M, Williamson P, Kirkham J, Kolamunnage-Dona R (2014) A review of the handling of missing longitudinal outcome data in clinical trials. Trials 15:1–11. https://doi.org/10.1186/1745-6215-15-237
van der Graaf JW, Kroeze RJ, Buckens CFM, Lessmann N, van Hooff M (2023) MRI image features with an evident relation to low back pain: a narrative review. Eur Spine J 32:1830–1841. https://doi.org/10.1007/s00586-023-07602-x
Wing RR, tate espeland DF, Lewis MA, Gokee LaRose CE, Gorin J, Bahnson AA, Perdue J, Hatley LH, Ferguson KE, Garcia E, Lang KR W (2016) Innovative self-regulation strategies to reduce weight gain in young adults. The study of novel approaches to weight gain prevention (SNAP) randomized clinical trial. JAMA Intern Med 176:755–762. https://doi.org/10.1001/jamainternmed.2016.1236
Roberts KE, Beckenkamp PR, Ferreira ML, Ho EK, Carvalho-e-Silva AP, Calais-Ferreira L, Ferreira PH (2023) The impact of aggregate positive lifestyle behaviors on low back pain resilience and care seeking. Spine J 23:1405–1413. https://doi.org/10.1016/j.spinee.2023.06.388
Acknowledgements
The authors would like to thank all the participants of this long-term follow-up study. The authors gratefully acknowledge the contribution of Tommi Jauhiainen, MD, in performing the MRI examinations.
Funding
This work was supported by Research Institute Orton, Orton Orthopaedic Hospital, Helsinki, Finland, and Helsinki University Hospital, Helsinki, Finland, through grants by the Ministry of Social Affairs and Health in Finland, and by the Siviä Kosti Foundation, Helsinki, Finland.
Open Access funding provided by University of Helsinki (including Helsinki University Central Hospital).
Author information
Authors and Affiliations
Contributions
Conseptualisation: Dietrich Schlenzka, Teija Lund. Data curation: Leena Ristolainen. Formal analysis: Martina Lohman, Dietrich Schlenzka, Hannu Kautiainen, Leena Ristolainen, Teija Lund. Funding acquisition: Dietrich Schlenzka, Teija Lund. Investigation: Anni Aavikko. Methodology: Martina Lohman, Dietrich Schlenzka, Teija Lund. Project administration: Leena Ristolainen. Resources: Leena Ristolainen. Writing – original draft: Anni Aavikko.Writing – review and editing: all authors. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This study was conducted according to the Declaration of Helsinki for research on human participants. The study protocol was approved by the Institutional Review Board, and ethical approval was granted by the Ethics Committee of Helsinki University Hospital on December 9, 2020 (protocol code 3251/2020).
Consent to participate
All participants gave their written informed consent prior to their involvement in the study.
Conflict of interest
No conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aavikko, A., Ristolainen, L., Kautiainen, H. et al. Relationship of disc degeneration after pubertal growth spurt to future low back pain: a longitudinal cohort study. Eur Spine J (2024). https://doi.org/10.1007/s00586-024-08366-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00586-024-08366-8