Abstract
Most diseases of the spine disproportionately impact older persons, with the modal (i.e., commonest) patient a female in their 8th decade of life. We examined the corpus of spinal RCTs to determine how many included “average” spine patients. We searched PubMed for randomized clinical trials published in the top 7 spine journals over a period of 5 years from 2016 to 2020 and extracted nominal upper age cut-offs and the distribution of ages actually recruited. We identified 186 trials of 26,238 patients. We found that only 4.8% of trials could be applied to an “average” 75-year-old patient. This age-based exclusion was not dependent on funding source. Age-based exclusion was exacerbated by explicit upper age cut-offs, however, the age-based exclusion went beyond explicit age cut-offs. Only few trials were applicable to older patients even amongst trials with no age cut-off specified. Age-based exclusion from clinical trials starts at late middle age. The mismatch between spinal patient’s age seen in clinical practice and spinal patient’s age in trials was so severe that over the 5 years (2016–2020) almost no RCT evidence was produced applicable to the “average” aged-patient across the body of literature available. In conclusion, age-based exclusion is ubiquitous, multifactorial, and happens on a supratrial level. Eliminating age-based exclusion involves more than an arbitrary lifting of explicitly stated upper age cut-offs. Instead, recommendations include increasing input from geriatricians and ethics committees, establishing updated or new models of cares, and creating new protocols to facilitate further research.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
“Evidence based medicine (EBM) is the conscientious, explicit, judicious and reasonable use of modern, best evidence in making decisions about the care of individual patients” [1]. This ensures that the best research is integrated with clinical practice to provide optimum patient care. Simply put, EBM requires an evidence base.
The Australian Spine Registry published an annual report in 2019, identifying that the age group of highest prevalence requiring spinal surgery was 70–79 years old [2]. This is unsurprising as ageing is a natural and inevitable process which is time-related and leads to the deterioration of necessary physiological functions for survival [3]. Specifically in the spine field, degenerative changes due to age alone were found in the spines of over 80% of patients over a period of 10 years, this was independent of other factors such as gender, smoking, and body mass index [4]. In addition to the increased comorbidities associated with age, a significant body of literature has demonstrated that older adults have worse post-operative complications and poorer outcomes following spinal interventions [5,6,7,8,9]. Therefore, as convenient as it would be, trial data from younger patients must not be applied to older patients as the external validity of these trials would be compromised.
Although ageism has been studied in other areas of medicine, within the spine field, there has only been one systematic review to our knowledge. Paeck et al. [10] conducted a systematic review with meta-analysis specifically looking at the exclusion of older adults from lower back pain randomised controlled trials (RCTs). Approximately 41% of RCTs from 1992 to 2010 excluded people over the age of 65-years, and the mean age of participants was 44.3 years old. This study was, however, conducted in 2014, and the authors acknowledged that one of the limitations was that they did not categorise exclusion of participants into age-groups, but rather, just divided studies into whether or not they had an upper age limit i.e., explicit exclusion.
Other studies included qualitative questionnaire-based studies, seeking opinions of health professionals, where majority agreed that older adults were often excluded from clinical trials [11, 12]. Ludmir et al. [13] also studied the age disparities in oncology trials by comparing the median ages of participants in the trials, and the median age of the actual target population. Although they did not look specifically at the exclusion of the ageing population, they noted that in all trials, the median age of participants in clinical trials were on average 6.49-years younger than the actual population age. There was also a significantly greater difference in median age in industry sponsored trials.
Moreover, in a systematic review conducted by Bourgeois et al. [14], it was concluded that elderly people were excluded from RCTs for drugs for ischaemic heart diseases. The type of funding of RCTs were also extracted in the process. RCTs with upper age cut-offs were more likely to be funded by non-industry sources.
The main gaps identified included the comparison of trials with an explicit upper age cut-off to trials that did not have an age cut-off. By doing this, explicit exclusion has been identified, however this does not translate to the applicability of evidence. Applicability of research goes beyond the explicitly stated upper age cut-offs. For example, a study with an upper age cut-off of 100-years-old might have participants with a median age of 40-years-old, with it’s 75th percentile age being 60-years-old. This means that 25% of less of the participants are 60-years-old and above, making it highly unapplicable for anyone over 60-years-old. Most studies also did not categorise exclusion of older adults by age groups, making it difficult to comprehend the extent of ageism.
Hence, we sought to understand the extent of ageism—the exclusion of a demographic based solely on their age—within the spinal research field by calculating how applicable the current evidence base was for each age group, bearing in mind that the patients with the age group of highest prevalence receiving spinal intervention were 70–79-years-old [2]. Our hypothesised reasons for age-based exclusion included the presence of upper age cut-offs when recruiting participants for trials, as well as the effect of industry sponsorship on trial design.
Methods
Framework
This research took the form of a systematic review [15]. Hence, Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines [16] were followed as applicable.
Informed consent was not obtained because no individual patient data was collected and we performed a meta-analysis only of publicly available data.
Eligibility criteria
Our eligibility criteria included any RCTs published from the 1/1/2016 to the 31/12/2020, studies published only in the English language, and published in the 7 spine journals most commonly used by spinal research professionals [17]—European Spine Journal, Spine, The Spine Journal, Global Spine Journal, Journal of Neurosurgery, Journal of Spinal Disorders Techniques, and Asian Spine Journal. Full texts were screened and studies containing any paediatric patients (under 18 years-old), studies that were not RCTs, and RCTs on animals and cadavers were excluded.
Search strategy
The search was performed in PubMed on the 23/03/2021 with the search strategy [“randomized controlled trial” (Publication Type) AND (“2016”[PDAT]:“2020”[PDAT])] AND (((((“Spine”[Journal] OR “European spine journal: official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research So0ciety”[Journal]) OR “The spine journal: official journal of the North American Spine Society”[Journal]) OR “Journal of neurosurgery. Spine”[Journal]) OR “Asian spine journal”[Journal]) OR “Global spine journal”[Journal]).
Data extraction
Dual extraction of the data was carried out by two members of the research team. Data identified included any explicit age cut-offs or nominal upper age, mean age, standard deviation of the mean age, median age, interquartile range, maximum age recruited, sponsorship type of research, and a broad classification of the intervention type. The mean age of the entire research population was extracted, however, if it was not provided, the mean age of the intervention group was chosen. The type of sponsorship was divided into three categories—industry sponsored, publicly sponsored, and no sponsorship. Classification of intervention types were divided into implants, surgery, interventional radiology, pharmacology, psychology and cognitive therapy, physiotherapy, bracing, and other. Any discrepancies between the dual extraction were resolved by adjudication by a third reviewer not involved in the extraction process.
Data transformation
The number of applicable trials were calculated for each 5-year age category from 40 to 100 years old. Trials were considered applicable if 25% or more of patients in the trial were of the patient’s age or older. This was determined by the using any given 75th percentile ages. If this was not provided, a 75th percentile age was extrapolated from the implied normal distribution using the mean age and standard deviation. This was calculated by the formula:
For secondary outcomes, the mean ages of patients in clinical trials with explicit upper age cut-offs were compared with those that did not have an upper age cut-off. The mean ages of patients in clinical trials with industry sponsorship were compared with public sponsorship as well as no sponsorship.
Data analysis and presentation
Data was analysed using SPSS Statistics and Microsoft Excel and will be presented primarily with estimation statistics and confidence interval testing. Data will be presented on heat maps, Gardner–Altman plots, and Cumming plots. Null-hypothesis significance testing—Chi-square and t-tests—will also be used.
Results
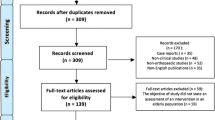
In total, 228 records were identified with the search strategy on PubMed. Full text articles were screened and assessed for eligibility, in which 42 were excluded due to the eligibility criteria. 186 studies were included with a total of 26,238 participants. Of the remaining 186 studies, 75th percentiles were obtained or extrapolated from 146 studies, which were used as our primary outcomes. Of the 186 studies, 173 studies had means which were used to for the secondary outcomes. 94 out of the 186 studies, approximately 50%, had explicit age cut-offs.
7 out of 186 trials explicitly stated that they excluded participants based solely on an arbitrary upper age limit (Fig. 1).
Primary outcomes
Primary outcomes are displayed in the form of a heatmap in Figs. 2 and 3, where the values in the figures are expressed as a percentage. Figure 2 demonstrates the number of studies applicable with the denominator excluding studies without a 75th percentile, while Fig. 3 demonstrates the number of studies applicable with the denominator including all studies, including those without a 75th percentile. The highest percentage, 100%, is shaded green and the lowest percentage, 0%, is shaded red.
Our findings demonstrate that older adults are at a higher risk of being excluded from RCTs. The overall trend observed was that as participant age increased, the number of relevant clinical trials decreased (Figs. 2, 3). This trend was evident in all intervention types. Overall, approximately 55% of studies were applicable to a 55-year-old individual, 6% of trials apply to a 75-year-old, and no trials were applicable to an 85-year-old individual. The implant intervention had the highest rate of exclusion, with no trials applicable to a 75-year-old individual.
Figure 3 exhibits a higher rate of exclusion in each 5-year age category. The denominator of the values in Fig. 3 included all trials, including those that we were not able to extrapolate a 75th percentile from. Approximately 44% of studies were applicable to a 55-year-old individual, 5% of trials were applicable to a 75-year-old individual, and no trials were applicable to anyone aged 85 and older.
Secondary outcomes
Explicit age cut-offs
84 out of the 173 studies (48%) with means had explicit age cut-offs. The mean age of patients in trials with cut-off ages and no cut-off ages were compared. Clinical trials with explicit age cut-offs were more likely to have a younger mean age. The data is represented in a Gardner–Altman estimation plot (Fig. 4). Participants in clinical trials with a cut-off age had a mean age of 46.8 years old, while participants in clinical trials without a cut-off age had a mean age of 55.9 years old. The mean difference was 9.1 years old with a 95% confidence interval of 6.2 and 11.8. The P value of the t-test was 0.0, the difference was statistically significant.
Gardner–Altman estimation plot displaying the mean difference between trials with cut-off ages and trials with no cut-off ages. Each trial is depicted as a dot on the left axes, and the mean difference is depicted as a dot on the floating axes on the right as a bootstrap sampling distribution. The 95% confidence interval is depicted by the ends of the vertical error bar
Sponsorship
Out of the 173 trials with mean ages, 55 had industry sponsorship (31.8%), 63 public sponsorship (36.4%), and 55 had no sponsorship (31.8%). The mean age of participants in clinical trials with public sponsorship and no sponsorship were compared against industry sponsored clinical trials. The type of sponsorship did not affect the mean ages of the clinical trials. The data is displayed in a Cumming estimation plot (Fig. 5). The mean ages for industry sponsored, publicly sponsored, and unsponsored clinical trials were 50.8, 50.7, and 53.0 years respectively. The mean difference between industry and publicly sponsored clinical trials was 0.1 with a 95% confidence interval of − 4.1 and 3.63. The mean difference between industry sponsored and unsponsored trials was 2.14 with a 95% confidence interval of − 2.1 and 6.24. The P values of the tests were 0.96 and 0.31 respectively. The difference was not statistically significant.
Cumming estimation plot comparing publicly sponsored and industry sponsored clinical trials against unsponsored clinical trials. The upper axes demonstrate the raw data, and the lower axes demonstrate the mean differences depicted as a dot and plotted as bootstrap sampling distributions. The 95% confidence interval is depicted by the ends of the vertical error bar
Fisher’s exact test was conducted on SPSS. The P value was 0.116—the type of sponsorship did not affect the likelihood of an explicit cut-off age in clinical trials (Table 1).
Discussion
Our primary results identified the lack of applicability of most spinal clinical trials for the ageing population. There was a decrease in applicability of spinal research with an increased age. The 75th percentile was used as a threshold for determining applicability, as it allows flexibility for normally and not normally distributed data, given that non-normal data is usually presented with an interquartile range. Figure 3 was created in addition to Fig. 2 as it expresses the true percentage of research applicable to an individual if they require treatment. Because the denominator for Fig. 3 also includes where trials cannot be applied because inadequate information is given to determine the spread of participant ages, it gives a stronger indication of evidence applicability as published.
Estimation statistics was primarily used to illustrate our secondary outcomes as overreliance on null-hypothesis significance testing—an accept or reject dichotomy—can divert attention of readers from the quantification of effect [18]. As hypothesised, clinical trials with explicitly stated cut-off ages had a significantly younger group of participants. While explicit age cut-offs do influence the participant ages, this only captures and explains a small part of exclusion of older adults, as less than half of the trials have explicit age cut-offs.
Industry sponsored trials in spine research have been shown to have an odds ratio of 3.3 times higher positive outcomes [19]. One of the potential reasons for this includes bias in study design, including the selection criteria of participants. Hence, sponsorship types were extracted and the mean ages in each sponsorship type was compared. Contrary to our hypotheses, the type of sponsorship did not affect the mean ages of participants in RCTs.
Upon examination of the results, we conclude that exclusion goes beyond explicit age cut-offs and sponsorship. Exclusion exists in three different layers—explicit exclusion, implicit exclusion, and systemic exclusion. Explicit exclusion of older adults is most noticeable, with a clearly stated age exclusion criteria. Implicit exclusion is more insidious—it is when there is no explicit exclusion, however, participants in the clinical trials do not match the research question. Essentially, very few older patients are actually included, even when they are technically not excluded. This was very obviously demonstrated by Ludmir et al. [13] when median ages of research participants were compared to median ages of patients in his study. Explicit and implicit exclusion applies to individual trials. A third layer of exclusion was identified in this study—systemic exclusion. This is demonstrated in Fig. 6, where the research question might match the research population, however, there is a mismatch with the actual population. Research as a whole is targeted towards diseases impacting younger people. In essence, there are discrepancies in where research efforts are going. Systemic exclusion goes beyond the level of individual trials and arises at a supra-trial level. Our results from this study indicate that systemic exclusion exists in the spine field.
Reasons for the exclusion of the elderly range from logistical factors to patient and family related factors. These include difficulty in accessing hospitals and transportation, the requirement of technology in many newer trials, and compliance with the suggested intervention [20, 21]. Furthermore, age-related hearing loss is not uncommon in the older population, which can create a barrier in communication [22]. Older adults are also at a higher risk of cognitive decline [23] which can affect their capacity when obtaining informed consent.
Older adults are more prone to multimorbidity, “the simultaneous occurrence of two or more diseases that may or may not share a casual link” [24]. Doctors may exclude participants due to concerns of unexpected side effects and intolerance of the treatment.
Supra-trial reasons for exclusion include resistance to change, an absence of models to follow, and a lack of adequate resources [25].
As describe previously, the ageing population is increasing and there has been a shift in epidemiology. Especially within the spine field, the marginalisation of older people in trials must be eliminated as many spinal conditions are age-related. Interventions used on younger adults may have differing efficacies for older adults. Although rates of complications are not expected to be identical, this information is critical for patient decision making. Chang et al. [26] conducted a systematic review with 7 million participants in 2020 and found that worse health outcomes were found in 95.5% of studies that excluded the elderly. Strong evidence was also found between ageism and elderly mistreatment, including physical abuse, financial abuse, sexual abuse, psychological/emotional abuse, and neglect [27].
Aside from the ethical concerns that arise from that, poor health outcomes lead to inevitable consequences—individuals aged 60 years or older account of 23% of the global burden of disease [28], and age-related diseases account of 51.3% of global burden among adults in 2017 [29]. In the United States of America, data from the Medicare Current Beneficiary Survey shows that medical expenses double between 70 and 90-year-olds, and that 52% of medical expenses stems from the top 10% of spenders [30]. In China, a country with an increasing ageing population, the medical expense of the ageing population per capita was almost three times more than those under 65-years of age [31].
While certain elderly individuals will need to be excluded due to certain comorbidities for health and safety reasons, this should not hinder research in the ageing population. Exclusion due to age alone for convenience is unethical. Researchers should never exclude participants solely based on an arbitrary age limit. Inclusion is essential.
To prevent ageism, it is unacceptable to just lift the upper age limit. Rather, targeted and specific research should be conducted for the elderly. On an individual trial basis, geriatricians should have an increased input in interventions as they are in a strong position to reduce unethical discrimination [32]. Researchers should also eliminate any bias as this can cause implicit exclusion. Ethics committees should request for justification for any explicit age cut-offs when looking at the methods of a study. Although these are small interventions, any small-scale intervention has been shown to yield a positive result [33].
However, as identified in this study, exclusion occurs on a supra-trial level. Table 2 identifies the roles of different bodies in preventing exclusion. While sponsorship did not have a significant impact, more funding should be provided towards trials for the ageing population as this encourages and provides incentives for research towards this group. More models of care and research protocols specific to the older population should be created and publicised to facilitate future research. Finally, we identify that more meta research should be undertaken to examine the impact of trial age populations across individual interventions. Ultimately, the end goal is to achieve optimal patient care.
References
Masic I, Miokovic M, Muhamedagic B (2008) Evidence based medicine—new approaches and challenges. Acta Inform Med 16:219–225. https://doi.org/10.5455/aim.2008.16.219-225
E Apos CJ, Ahern S, Truong T, Hansen J, Johnson MA (2021) The Australian Spine Registry Annual Report, 2020. In: Spine Society of Australia and School of Public Health and Preventive Medicine, Monash Universit.
SF G (2000) Aging: the biology of senescence. In: Developmental biology. Sinauer Associates, Sunderland
Andersson GBJ (1998) 8 what are the age-related changes in the spine? Baillieres Clin Rheumatol 12:161–173. https://doi.org/10.1016/S0950-3579(98)80010-1
Cloyd JM, Acosta FL Jr, Cloyd C, Ames CP (2010) Effects of age on perioperative complications of extensive multilevel thoracolumbar spinal fusion surgery. J Neurosurg Spine 12:402–408. https://doi.org/10.3171/2009.10.Spine08741
Kobayashi K, Imagama S, Ando K, Ishiguro N, Yamashita M, Eguchi Y, Matsumoto M, Ishii K, Hikata T, Seki S, Terai H, Suzuki A, Tamai K, Aramomi M, Ishikawa T, Kimura A, Inoue H, Inoue G, Miyagi M, Saito W, Yamada K, Hongo M, Nishimura H, Suzuki H, Nakano A, Watanabe K, Chikuda H, Ohya J, Aoki Y, Shimizu M, Futatsugi T, Mukaiyama K, Hasegawa M, Kiyasu K, Iizuka H, Iizuka Y, Kobayashi R, Nishida K, Kakutani K, Nakajima H, Murakami H, Demura S, Kato S, Yoshioka K, Namikawa T, Watanabe K, Nakanishi K, Nakagawa Y, Yoshimoto M, Fujiwara H, Nishida N, Imajo Y, Yamazaki M, Sakane M, Abe T, Fujii K, Kaito T, Furuya T, Orita S, Ohtori S (2017) Complications associated with spine surgery in patients aged 80 years or older: Japan Association of Spine Surgeons with Ambition (JASA) Multicenter Study. Glob Spine J 7:636–641. https://doi.org/10.1177/2192568217716144
Kobayashi K, Imagama S, Sato K, Kato F, Kanemura T, Yoshihara H, Sakai Y, Shinjo R, Hachiya Y, Osawa Y, Matsubara Y, Ando K, Nishida Y, Ishiguro N (2018) Postoperative complications associated with spine surgery in patients older than 90 years: a multicenter retrospective study. Glob Spine J 8:887–891. https://doi.org/10.1177/2192568218767430
Wang MY, Widi G, Levi AD (2015) The safety profile of lumbar spinal surgery in elderly patients 85 years and older. Neurosurg Focus FOC 39:E3. https://doi.org/10.3171/2015.7.FOCUS15180
Bank M, Gibbs K, Sison C, Kutub N, Paptheodorou A, Lee S, Stein A, Bloom O (2018) Age and other risk factors influencing long-term mortality in patients with traumatic cervical spine fracture. Geriatr Orthop Surg Rehabil. https://doi.org/10.1177/2151459318770882
Paeck T, Ferreira ML, Sun C, Lin CW, Tiedemann A, Maher CG (2014) Are older adults missing from low back pain clinical trials? A systematic review and meta-analysis. Arthritis Care Res (Hoboken) 66:1220–1226. https://doi.org/10.1002/acr.22261
van Marum RJ (2020) Underrepresentation of the elderly in clinical trials, time for action. Br J Clin Pharmacol 86:2014–2016. https://doi.org/10.1111/bcp.14539
Crome P, Lally F, Cherubini A, Oristrell J, Beswick AD, Clarfield AM, Hertogh C, Lesauskaite V, Prada GI, Szczerbińska K, Topinkova E, Sinclair-Cohen J, Edbrooke D, Mills G (2011) Exclusion of older people from clinical trials: professional views from nine European countries participating in the PREDICT study. Drugs Aging 28:667–677. https://doi.org/10.2165/11591990-000000000-00000
Ludmir EB, Mainwaring W, Lin TA, Miller AB, Jethanandani A, Espinoza AF, Mandel JJ, Lin SH, Smith BD, Smith GL, VanderWalde NA, Minsky BD, Koong AC, Stinchcombe TE, Jagsi R, Gomez DR, Thomas CR Jr, Fuller CD (2019) Factors associated with age disparities among cancer clinical trial participants. JAMA Oncol 5:1769–1773. https://doi.org/10.1001/jamaoncol.2019.2055
Bourgeois FT, Orenstein L, Ballakur S, Mandl KD, Ioannidis JPA (2017) Exclusion of elderly people from randomized clinical trials of drugs for ischemic heart disease. J Am Geriatr Soc 65:2354–2361. https://doi.org/10.1111/jgs.14833
Grant MJ, Booth A (2009) A typology of reviews: an analysis of 14 review types and associated methodologies. Health Info Libr J 26:91–108. https://doi.org/10.1111/j.1471-1842.2009.00848.x
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71. https://doi.org/10.1136/bmj.n71
Martin JT, Gullbrand SE, Fields AJ, Purmessur D, Diwan AD, Oxland TR, Chiba K, Guilak F, Hoyland JA, Iatridis JC (2018) Publication trends in spine research from 2007 to 2016: comparison of the Orthopaedic Research Society Spine Section and the International Society for the Study of the Lumbar Spine. JOR Spine 1:e1006. https://doi.org/10.1002/jsp2.1006
Ho J, Tumkaya T, Aryal S, Choi H, Claridge-Chang A (2019) Moving beyond P values: data analysis with estimation graphics. Nat Methods 16:565–566. https://doi.org/10.1038/s41592-019-0470-3
Shah R, Albert T, Bruegel-Sanchez V, Vaccaro A, Hilibrand A, Grauer J (2005) Industry support and correlation to study outcome for papers published in spine. Spine 30:1099–1104. https://doi.org/10.1097/01.brs.0000161004.15308.b4. (Discussion 1105)
Voulgaris E, Vomvas D, Kesisis G, Karampeazis A, Pallis A (2010) Why are older patients excluded from clinical trials? Forum Clin Oncol 1:42–46
Helfand BKI, Webb M, Gartaganis SL, Fuller L, Kwon C-S, Inouye SK (2020) The exclusion of older persons from vaccine and treatment trials for coronavirus disease 2019—missing the target. JAMA Intern Med 180:1546–1549. https://doi.org/10.1001/jamainternmed.2020.5084
National Instituteon Deafnessand Other Communication Disorders (2018) Age-related hearing loss. https://www.nidcd.nih.gov/health/age-related-hearing-loss
Alzheimer’s Association (2014) 2014 Alzheimer’s disease facts and figures. Alzheimers Dement 10:e47-92. https://doi.org/10.1016/j.jalz.2014.02.001
Fabbri E, Zoli M, Gonzalez-Freire M, Salive ME, Studenski SA, Ferrucci L (2015) Aging and multimorbidity: new tasks, priorities, and frontiers for integrated gerontological and clinical research. J Am Med Dir Assoc 16:640–647. https://doi.org/10.1016/j.jamda.2015.03.013
Watts G (2012) Why the exclusion of older people from clinical research must stop. BMJ:Br Med J 344:e3445. https://doi.org/10.1136/bmj.e3445
Chang ES, Kannoth S, Levy S, Wang SY, Lee JE, Levy BR (2020) Global reach of ageism on older persons’ health: a systematic review. PLoS One 15:e0220857. https://doi.org/10.1371/journal.pone.0220857
Pillemer K, Burnes D, MacNeil A (2021) Investigating the connection between ageism and elder mistreatment. Nat Aging 1:159–164. https://doi.org/10.1038/s43587-021-00032-8
Prince M, Wu F, Guo Y, Gutiérrez-Robledo L, O’Donnell M, Yusuf S (2014) The burden of disease in older people and implications for health policy and practice. Lancet. https://doi.org/10.1016/S0140-6736(14)61347-7
Chang AY, Skirbekk VF, Tyrovolas S, Kassebaum NJ, Dieleman JL (2019) Measuring population ageing: an analysis of the Global Burden of Disease Study 2017. Lancet Public Health 4:e159–e167. https://doi.org/10.1016/S2468-2667(19)30019-2
De Nardi M, French E, Jones JB, McCauley J (2016) Medical spending of the US elderly. Fisc Stud 37:717–747. https://doi.org/10.1111/j.1475-5890.2016.12106
Yang Y, Zheng A, Li M, Duan W, Mu X, Wang X (2016) Medical economic burden of the ageing population: a multistage sampling analysis of 3 532 517 cases. Lancet 388:S79. https://doi.org/10.1016/S0140-6736(16)32006-2
Briggs R, Robinson S, O’Neill D (2012) Ageism and clinical research. Ir Med J 105:311–312
Burnes D, Sheppard C, Henderson CR Jr, Wassel M, Cope R, Barber C, Pillemer K (2019) Interventions to reduce ageism against older adults: a systematic review and meta-analysis. Am J Public Health 109:e1–e9. https://doi.org/10.2105/AJPH.2019.305123
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
conflict of interest
There are no conflicts of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chew, G., Menounos, S., Sheldrick, K. et al. Age-based exclusion is common and multifactorial in spinal RCTs: a systematic review and quantitative analysis. Eur Spine J 32, 1537–1545 (2023). https://doi.org/10.1007/s00586-023-07618-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-023-07618-3