Abstract
Purpose
To compare the residual range of motion (ROM) of cortical screw (CS) versus pedicle screw (PS) instrumented lumbar segments and the additional effect of transforaminal interbody fusion (TLIF) and cross-link (CL) augmentation.
Methods
ROM of thirty-five human cadaver lumbar segments in flexion/extension (FE), lateral bending (LB), lateral shear (LS), anterior shear (AS), axial rotation (AR), and axial compression (AC) was recorded. After instrumenting the segments with PS (n = 17) and CS (n = 18), ROM in relation to the uninstrumented segments was evaluated without and with CL augmentation before and after decompression and TLIF.
Results
CS and PS instrumentations both significantly reduced ROM in all loading directions, except AC. In undecompressed segments, a significantly lower relative (and absolute) reduction of motion in LB was found with CS 61% (absolute 3.3°) as compared to PS 71% (4.0°; p = 0.048). FE, AR, AS, LS, and AC values were similar between CS and PS instrumented segments without interbody fusion. After decompression and TLIF insertion, no difference between CS and PS was found in LB and neither in any other loading direction. CL augmentation did not diminish differences in LB between CS and PS in the undecompressed state but led to an additional small AR reduction of 11% (0.15°) in CS and 7% (0.05°) in PS instrumentation.
Conclusion
Similar residual motion is found with CS and PS instrumentation, except of slightly, but significantly inferior reduction of ROM in LB with CS. Differences between CS and PS in diminish with TLIF but not with CL augmentation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Posterolateral pedicle screw (PS) instrumentation is the gold standard in spinal fusion surgery, whose success largely relies on the residual motion of the instrumentation construct [1, 2]. Cortical screws (CS), otherwise known as the cortical bone trajectory (CBT), were introduced in 2009 by Santoni et al. [3]. CS follow a trajectory that increases cortical bone purchase, leading to improved screw hold in the lumbar spine. As compared to the traditional PS, which typically follows a converging path along the axis of the pedicle, CS begin at the lateral part of the pars interarticularis and follow a caudocephalad diverging track (Fig. 1). The medial screw entry points enable a less invasive approach for posterior instrumentation by limiting the dissection of the superior facet joints and reducing paraspinal muscle dissection and retraction [4]. In several biomechanical analyses, CS outperformed PS in insertion torque, toggling, and pull-out strength [3, 5, 6].
The original intention for the use of CS was in the setting of an osteoporotic lumbar spine, in which the CS obtain enhanced cortical fixation [3]. Soon after its first description, the CS technique was expanded to the lower thoracic spine (T9-12) and sacrum [7, 8]. Indications for CS were further broadened to include trauma surgery and adjacent segment disease, as well as it being used as a rescue option for loose or misplaced PS [9].
Although CS may improve screw purchase, some concerns have emerged regarding possibly increased residual motion of the CS construct due to the short lever arm from the median axis to the more medial running screw heads and vertical rods [9]. Some authors have reported decreased stiffness against lateral bending and axial rotation loadings with CS as compared to PS instrumented constructs [10, 11]. However, no study has comparatively investigated residual motion in these constructs before and after decompression surgery to date. Therefore, the true difference in performance of CS vs PS in regard to residual motion are still unknown for may clinical scenarios, especially in the setting of degenerative disease.
To decrease redidual motion and counteract the potential biomechanical drawbacks of CS, several authors have advocated the insertion of large interbody fusion cages to reconstruct anterior column support and the use of crosslinks (CL) bilaterally attached to the vertical rods [9,10,11]. However, the effect of interbody fusion in CS constructs has not been investigated to date. Likewise, the benefit of CL augmentation is still unclear, as only trends but non-significant ROM differences were found between CS and PS on intact thoracic and lumbar segments in a previous, small sample size study [12].
The purpose of the current study was to compare the residual motion of CS versus PS instrumented lumbar segments before and after decompression surgery. The secondary aims were to evaluate the effects of (1) transforaminal interbody fusion (TLIF) and (2) CL augmentation on residual motion of CS and PS instrumentations.
Methods
Specimen preparation and instrumentation
This study was approved by the local ethics committee (BASEC Nr. 2017-00874). Twelve fresh-frozen human spine cadavers (Science Care, Phoenix, AZ, USA) with an average age of 59 years (range 50–68; eight males and four females) were used for this study. 11 cadavers were caucasian, 1 of unspecified race. Height, weight and BMI was 171 cm (157–196 cm), 90 kg (45–108 kg) and 27.9 (16.1–37.3). Houndsfield measurements in vertebral body L3 was 201 (range 173–245) [13]. No further information from the deceased regarding osteoporosis and bone quality were available. The specimens did not contain any osseous defects or deformities as assessed on computed tomography scans and magnetic resonance imaging. After thawing, the cadavers were dissected into the segments L1–L2, L3–4, and L5–S1, leading to a total of 36 (12 × 3) specimens. The specimens were denuded of the surrounding muscle and connective tissue without harming the intersegmental ligamentous structures, facet joints, or intervertebral discs. Following the initial dissection, the specimens were again stored at − 20 °C until instrumentation, mounting, and biomechanical testing. Biomechanical testing was performed after thawing overnight at room temperature of 20 °C. During biomechanical testing, a vertebral body fracture occurred in one PS instrumented L5/S1 specimen after TLIF insertion, after which the segment lost its hold in the clamp and was not testable anymore. The specimen was excluded, leaving a total of 35 segments eligible for analysis.
Segmental instrumentation
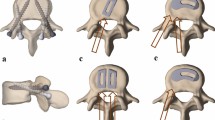
With the obtained CT data, triangular surface models of the vertebrae were generated. We used an in-house developed computer-aided design (CAD) surgical planning software to plan and execute all pedicle screw trajectories with patient specific instruments (PSI). 2.7 mm PSI drill guides were 3D-printed and used for instrumentation with cannulated titanium alloy poly-axial pedicle screws (Medacta International, Castel San Pietro, Switzerland) [14, 15]. Half of the vertebral segments were planned and instrumented with CS, and the other half with the PS technique, each with optimized screw diameters (CS: 5.0–6.0 mm; PS 6.0–7.0 mm) and lengths (CS 40–50 mm; PS 50–60 mm) (Fig. 2) [16, 17]. For sacral CS, the technique proposed by Matsukawa et al. [8] was used. However, the screw tips reached but did not penetrate the S1-endplate so as not to hinder the interbody cage placement in the later course. Pre-bent titanium rods were used to link the pedicle screws on each side vertically.
Cortical screw (CS) and pedicle screw (PS) instrumentation with patient specific instruments (PSI). a, b Instrumentation of L5/S1 segment with PSI-guided CS. Note the diverging and upwardly pointing screw trajectory. c, d Instrumentation of L1/2 segment with PSI-guided traditional converging PS trajectory parallel to the corresponding upper endplate
Residual range of motion (ROM) of the segments was tested with and without CL augmentation with one CL (straight cross connector, MUST Medacta International, Switzerland) and before and after midline decompression with bilateral laminotomy, unilateral facetectomy and insertion of a TLIF cage (MectaLIF Transforaminal, Medacta International, Switzerland) (Fig. 3). The supra- and interspinous ligaments were left intact when possible (Fig. 3d), but were resected in segments with tiny interspinous space, that did not allow the CL to be tunneled through (Fig. 3b). To only measure the effect of the implanted and connected hardware and not the removal of the supra- and interspinous ligaments, ROM was measured after dissecting these ligaments in the intact, uninstrumented segments. Accurate TLIF cage size (8–15 mm) was determined based on the previously acquired CT scans. Prior to cage implantation, a segmental distraction of − 100 N was applied to facilitate cage insertion. After cage insertion, an axial compression force of + 200 N was applied and the vertical rods tightened [18].
Biomechanical setup and tested decompression/instrumentation states. a Cortical screw (CS) instrumented L3/4 segment fixed in the test setup with 3D-printed mounting clamps. The segment is non-decompressed and non-fused as no vertical rods are attached. The range of motion was measured between the markers (*) attached to the cranial and caudal vertebrae. Additional markers (**) were installed on the cranial and caudal mounting clamps to control potential excess motions between the vertebrae and the test setup. b CS instrumented, non-decompressed segment without (left) and with (right) crosslink (CL) augmentation. c CS instrumented segment after bilateral laminotomy, unilateral facetectomy and transforaminal lumbar interbody fusion (TLIF). d L3/4 segment with traditional pedicle screws (PS) instrumentation, non-decompressed e after decompression, facetectomy and TLIF insertion
Biomechanical setup
The cranial and caudal vertebrae of the segments were each fixed with 3D-printed clamps [19, 20]. The vertebral bodies were installed with infrared-emitting markers, and additional markers were set on the clamps to control for excess movement between the vertebrae and the clamps. A biaxial linear-torsion testing protocol (Zwick/Roell Allroundline 10 kN and testXpert III Software, ZwickRoell GmbH & Co. KG, Germany) was used (Fig. 4). After five pre-conditioning cycles, the load-controlled ROM was recorded over one cycle with a motion-capture system set at 10 Hz and 0.09 mm accuracy (Fusion Track 500, Atracsys, Puidoux, Switzerland). Pre-defined loads were applied to the cranial vertebra, with the caudal vertebra fixed to the semi-constrained test rig. Flexion–extension (FE), lateral bending (LB), and axial rotation (AR) were recorded with a velocity of 1°/sec until ± 7.5 Nm. Anterior shear (AS) and lateral shear (LS) were recorded at 0.5 mm/sec and a predefined load of ± 150 N. Axial compression-decompression (AC) was recorded at 0.1 mm/sec until + 400 N compression and − 150 N distraction was reached [21].
Biomechanical setup. a Motion capture system in the top right records the range of motion. b Spinal segment in the vertical position for testing of axial rotation and axial compression. The caudal vertebra was attached to the semi-constrained test rig, which allowed free translational movements in the horizontal plane. The cranial and caudal vertebral bodies were each installed with a marker, and additional markers were set on the cranial and caudal 3D-printed mounting clamps
Statistical evaluation
Statistical evaluation was performed with MATLAB (Matlab 2019a, MathWorks, Massachusetts, USA). Shapiro–Wilk tests were conducted to assess the hypothesis of composite normality regarding the distribution of the data. The results suggested that some of the data were non-normally distributed. Therefore, medians are reported along with 25th and 75th percentiles in parenthesis and non-parametric statistical testing was performed. Independent samples tests (Mann–Whitney U) were used for the comparison of PS and CS instrumentation constructs in regard to residual motion and relative decreases of ROM. On the other hand, paired comparisons (Wilcoxon signed-rank) were performed to assess the effect CL on ROM in both the PS and CS instrumentation constructs. Because of multiple comparisons, the p-values were corrected with the Bonferroni. The significance level α was set to 0.05.
Results
In uninstrumented segments, similar residual motionlevels were documented in all loading directions between the CS and PS groups (Table 1; Fig. 5). Compared to the instrumented segments, all angular ROM values were significantly reduced with both CS and PS instrumentations, with FE being reduced the most, followed by LB and AR (all p < 0.05). Less, but also significant reduction of ROM were found in the translational loading directions AS and LS with both instrumentation techniques (p < 0.05). AC ROM was not affected by posterolateral fusion in either group.
In segments without interbody fusion, a significantly lower reduction in LB was found with CS instrumentation as compared to PS, with relative reductions of 61% (52–64%; absolute 3.3°) with CS and 71% (63–79%; absolute 4.0°) with PS (p = 0.048) (Table 1). This difference was unaffected by CL augmentation, with relative decreases of LB ROM of 60% (54–65%; absolute 3.1°) with CS and 72% (64–80%; absolute 4.0°) with PS (p = 0.016). The FE, AR, AS, LS, and AC values were similar and not statistically different between CS and PS instrumented, undecompressed segments, regardless of CL augmentation (Table 1).
After bilateral laminotomy, unilateral facetectomy, and TLIF, residual motion was similar and not statistically different between CS and PS instrumented segments in any ROM direction. The LB ROM of CS instrumented segments without CL was 67% (57–73%; absolute 3.5°), as compared to 75% (63–78%; absolute 3.5°) in the PS group, with this difference not being statistically significant.
CL augmentation led to a further significant decrease in ROM in AR in both CS and PS instrumented segments, but did not affect LB and only reduced FE in PS instrumented segments after decompression and TLIF (Fig. 6). In the CS group, usage of CL further reduced the AR ROM by 11% (8–15%; absolute 0.15 mm) and 18% (15–23%; absolute 0.31 mm) in undecompressed and decompressed segments with TLIF, respectively. In the PS group, CL reduced AR ROM by 7% (2–13%; absolute 0.05 mm) in undecompressed segments and 10% (7–16%; absolute 0.10 mm) after decompression and TLIF. FE ROM was slightly reduced in PS instrumented segments after decompression and TLIF by 3% (2–5%; absolute 0.04 mm), but FE was not affected by CL in CS undecompressed segments and CS instrumentation.
Discussion
This biomechanical study comparatively investigated residual motion following CS and traditional PS instrumentation before and after decompression, interbody fusion with TLIF and CL. The main findings are that adequate and similarly low levels of residual motion can be achieved with both screw trajectories, but that CS leads to a 10% inferior decrease in ROM in LB. Whereas TLIF insertion did compensate for this disadvantage in LB in CS constructs, CL augmentation did not.
The less invasive CS instrumentation technique is increasing in popularity [22]. Several biomechanical studies have shown favorable mechanical properties at the screw-bone interface with CS. In its first report, the CS technique demonstrated a 30% increase in failure load in uniaxial pullout tests as compared to PS in the cadaveric lumbar specimen (p = 0.08) [3]. Using quantitative CT-measurements, the above researchers further found a juxtaposition of higher quality bone in CS compared to PS and advocated CS use in patients with poor trabecular bone quality. Other authors found improved resistance to toggling tests, with CS requiring 184 cycles, as compared to 102 cycles with PS, to reach 2 mm of screw displacement [5]. In vivo measurements have further shown maximum insertional torques twice as high with CS as with PS, further indicating improved ultimate fixation strength with the CS technique [6]. However, screw-purchase with either technique was not in the focus of the present study, in which we had excluded spine specimens with decreased bone quality. We had rather aimed to investigate the differences in residual ROM after instrumentation with CS versus PS.
Concerns regarding construct stiffness with the CS technique remain ever since the below summarized findings of the biomechanical studies of Perez-Orribo et al. [10] and Matsukawa et al. [11]. The human cadaver study by Perez-Orribo et al. [10] found no significant differences between CS and PS in terms of ROM in any loading direction, with and without interbody support. However, after subdividing ROM in the angular lax and angular stiff zones, the study reported that PS, as compared to CS, had a significantly lower ROM in AR in specimen without interbody support and a significantly lower ROM in LB after TLIF insertion in the angular stiff zone. In the finite element study of Matsukawa et al. [11], improved screw pull-out strength and higher vertebral fixation strength on flexion (+ 51%) and extension (+ 35%) were found with CS as compared to PS, but lower fixation strength on lateral bending (− 20%) and axial rotation (− 37%). Based on the findings of these studies, several authors have advocated enhancing the CS instrumentation construct with interbody support and cross-connectors [9,10,11]
The present study is the first to comprehensively investigate the differences of CS and PS before and after decompression surgery, interbody fusion and CL-augmentation. In a previous study [12], we had conducted ROM measurements on six thoracic, one thoracolumbar and nine lumbar spine segments of four intact human cadaver spines and assessed the effect of CS and PS instrumentation with and without CL-augmentation. Although the study already provided some insight on the topic and showed some non-significant trends towards larger ROM in LB CS compared to PS instrumentation, the study failed to show any significant difference [12]. In consequence, no augmentation technique would be justified to counteract any non-present weakness in CS instrumentation. However, the previous study revealed some flaws and some questions were left unanswered, which we aimed to more concretely tackle with the present study. Firstly, the previous study may have potentially encompassed a type II error, attributable to the low sample size and the mixture of rather different thoracic segments and lumbar segments [12]. We aimed to more concretely address the concerns raised by Perez-Orribo et al. [10] and Matuskawa et al. [11] with a larger sample size (n = 35) restricted to the lumbar and lumbosacral spine only to uncover a potentially relevant difference between CS and PS. Secondly, the previous study [12] had investigated ROM and the effect of CL augmentation on intact segments only. Thus, whether these results are translatable to a clinical scenario including decompression surgery remained unclear. Thirdly, Matsukawa et al. [9] had not only suggested CL-augmentation but also interbody fusion to counteract potential biomechanical drawbacks of CS, which has not been scientifically investigated before. These factors prompted to conduct the present study with a larger sample size and limited to the lumbar and lumbosacral segments on both intact segments and decompressed segments with interbody fusion. By doing so, we aimed to draw more profound conclusions regarding differences in resdiual motion between CS and PS and the use of interbody fusion and CL augmentation.
The main findings of our study are that CS instrumentation provides similar levels of residual ROM to PS instrumentation in the lumbar spine in most ROM loading directions, but the present study proves a significant lower reduction of ROM of approximately 10% in LB with CS. This difference revealed significant in the present study, which we attribute to the larger sample size and the more uniform segments limited to the lumbar/lumbosacral spine. Concerns regarding shortcomings in AR in CS constructs could not be confirmed by our study. Based on our results, a reduction of approximately 40% in AR ROM can be expected with both CS and PS.
After decompression, unilateral facetectomy, and TLIF insertion, the ROM of CS and PS instrumented segments were equal, and the difference in LB diminished. The additional LB reduction by TLIF insertion may therefore be considered to promote bony fusion in CS constructs. However, whether the additional decrease in LB ROM with TLIF insertion of 6% warrants the routine use TLIF insertion in all CS constructs remains debatable. Although the clinical implications are speculative, it seems plausible that reduction of ROM is most important in directions with high native ROM to achieve a stiff construct and promote bony fusion. Therefore, we assume, that reductions of ROM are most important in FE, followed by LB, and less important in AR and translational movements. Hence, a 6% reduction in residual motion of the relatively large native LB ROM of 5.5° may have more clinical impact than a 6% decrease in a relatively smaller native AR ROM of 2.2°, for example. A recent systematic review [23] reported significantly increased fusion rates with different interbody fusion techniques compared to posterolateral instrumentation alone, with an OR for fusion with TLIF of 2.46. Interbody fusion has further shown to reduce stress on the pedicle screw-bone and screw-rod interfaces [24] and reduce the risk of early screw loosening by 60% [25]. In our opinion, interbody fusion should thus be considered in every lumbar fusion surgery and even more with CS instrumentation.
In our study, CL augmentation did not compensate for the differences in residual motion in LB between CS and PS. CL augmentation primarily led to a further decrease in AR motion, which tended to be higher in CS than PS instrumented segments, with an additional decrease of ROM in AR of approximately 11% versus 7% in undecompressed and 18% versus 10% in decompressed and TLIF instrumented segments, respectively. These findings are in line with previous studies [12, 18]. However, in light of the absolute decrease in ROM with CL augmentation of only 0.1°–0.3° in AR, we doubt that the routine use of CL augmentation pivotally improves bony fusion. Small and insignificant reduction in ROM with CL-augmentation have also been observed by Arand et al. [26]. In our opinion, the marginal additional effect in AR ROM with CL augmentation does not warrant the potential drawbacks such as increased surgical time and higher implant costs. However, regardless of the pedicle-screw trajectory, CL augmentation may be beneficial in highly unstable multi-level constructs and unstable three-column fractures [18].
With respect to reported clinical outcomes, Sakaura et al. [27] performed a retrospective study on patients treated with two-level lumbar fusion due to degenerative spondylolisthesis and found a decreased incidence of radiographic adjacent segment disease within 3 years after CS (13%) versus PS (42%). On the other hand, the same authors also found a decreased (p > 0.05) union rate with CS (91%) as compared to PS (95%) after a minimum follow-up of two years, which they attributed to a potentially lower segmental stiffness (i.e. higher residual motion) with the CS construct [28]. On the basis of these findings, the authors recommended using a CL to stiffen the cortical screw-posterior lumbar interbody fusion construct. In contrast, the prospective randomized trial by Lee et al. [29] did not find any difference in radiographic or patient-reported outcomes between CS and PS, with adequate and similar rates of fusion after a follow-up of two years. However, experiences and clinical results after CS instrumentation and the effect of CL and interbody fusion in these constructs are limited. Therefore, further clinical studies are needed to elucidate the long-term outcomes of the CS technique and verify the biomechanical findings of this study.
Limitations
This biomechanical study has certain limitations. The ROM measurements in isolated loading directions represent rough simplifications of human spinal motion patterns in vivo. In our opinion, the semi-constrained setup used in this study, provides well-defined loading conditions, that can easily be replicated on one hand and provide an appropriate simplification of the complex in-vivo loading conditions on the other hand [30]. This compromise between the exact replication of the physiological conditions and the reduction of complexity appears adequate to address the study questions, but some authors favor un-constrained test setups to allow coupled motions in all dimensions [21]. The residual motion reported in this study would best reflect the primary ROM of the segment shortly after the surgical procedure. However, how the different screw constructs perform in the long-term was not investigated within this study and would rather be evaluated with repetitive loading / fatigue analysis. The discussed study by Perez-Orribo et al. [10] differed between angular lax ROM ("portion of ROM in which ligaments/ hardware are lax") and angular stiff ROM ("portion of the ROM in which ligaments/hardware are under tension") and found less ROM reduction in AR in CS constructs in the angular stiff zones. In the here presented study, the instrumented segments did not show a biphasic load deflection curve (as it is typically observed in native spinal segments) but a largely constant stiffness along the whole range of motion. With that, the proposed methods for the division of the load–deflection curves into lax and stiff zone cannot be employed (50% slope technique) or define the whole ROM as the stiff-zone (zero-load extrapolation method) [31]. This effect can be explained by the assumption that the implanted hardware engages promptly at already minimal motion, essentially eliminating both neutral and lax zone. In consequence, subdividing ROM is not feasible and analyzing ROM appears sufficient to compare the different types of instrumentations.
In addition to large interbody grafting, Matsukawa et al. [9] have recommended preserving the facet joint as a further countermeasure against torsional stress, which we did not take into account herein. Thus, whether the residual motion of the CS-construct is dependent on the type of interbody implant and the preservation of the facet joint remains to be investigated. The value of CS instrumentation in osteoporotic bone has not been investigated in this study, as segments with decreased bone quality on CT were ruled out. Further, the CT of the specimen were performed without phantom and no DEXA measurements were available.
Conclusion
In intact lumbar segments, adequate and similarly low residual motion can be expected with CS and PS instrumentation in the lumbar spine, except of a slightly, but significantly inferior decrease of ROM in LB with CS. Concerns regarding residual motion in AR in CS constructs could not be confirmed by our study, which showed similar reduction of ROM in AR with both CS and PS. After midline decompression and TLIF, the residual motion of CS and PS instrumented segments were equal, and the difference in LB motion diminished. TLIF may therefore be considered to promote bony fusion in CS constructs. Whereas the use of CL revealed some additional decrease in AR ROM in both CS and PS constructs, it did not compensate the higher residual motion in LB in CS constructs. Therefore, the present data do not promote routine use of CL augmentation in CS instrumentation.
References
Zdeblick TA (1993) A prospective, randomized study of lumbar fusion. Prelim Results Spine 18:983–991. https://doi.org/10.1097/00007632-199306150-00006
Boos N, Webb JK (1997) Pedicle screw fixation in spinal disorders: a European view. Eur Spine J 6:2–18. https://doi.org/10.1007/bf01676569
Santoni BG, Hynes RA, McGilvray KC, Rodriguez-Canessa G, Lyons AS, Henson MA, Womack WJ, Puttlitz CM (2009) Cortical bone trajectory for lumbar pedicle screws. Spine J 9:366–373. https://doi.org/10.1016/j.spinee.2008.07.008
Matsukawa K, Kato T, Yato Y, Sasao H, Imabayashi H, Hosogane N, Asazuma T, Chiba K (2016) Incidence and risk factors of adjacent cranial facet joint violation following pedicle screw insertion using cortical bone trajectory technique. Spine 41:E851-e856. https://doi.org/10.1097/brs.0000000000001459
Baluch DA, Patel AA, Lullo B, Havey RM, Voronov LI, Nguyen NL, Carandang G, Ghanayem AJ, Patwardhan AG (2014) Effect of physiological loads on cortical and traditional pedicle screw fixation. Spine 39:E1297-1302. https://doi.org/10.1097/brs.0000000000000553
Matsukawa K, Yato Y, Kato T, Imabayashi H, Asazuma T, Nemoto K (2014) In vivo analysis of insertional torque during pedicle screwing using cortical bone trajectory technique. Spine 39:E240-245. https://doi.org/10.1097/brs.0000000000000116
Matsukawa K, Yato Y, Hynes RA, Imabayashi H, Hosogane N, Asazuma T, Matsui T, Kobayashi Y, Nemoto K (2017) Cortical bone trajectory for thoracic pedicle screws: a technical note. Clin Spine Surg 30:E497-e504. https://doi.org/10.1097/bsd.0000000000000130
Matsukawa K, Yato Y, Kato T, Imabayashi H, Asazuma T, Nemoto K (2014) Cortical bone trajectory for lumbosacral fixation: penetrating S-1 endplate screw technique: technical note. J Neurosurg Spine 21:203–209. https://doi.org/10.3171/2014.3.Spine13665
Matsukawa K, Yato Y (2017) Lumbar pedicle screw fixation with cortical bone trajectory: a review from anatomical and biomechanical standpoints. Spine Surg Relat Res 1:164–173. https://doi.org/10.22603/ssrr.1.2017-0006
Perez-Orribo L, Kalb S, Reyes PM, Chang SW, Crawford NR (2013) Biomechanics of lumbar cortical screw-rod fixation versus pedicle screw-rod fixation with and without interbody support. Spine 38:635–641. https://doi.org/10.1097/BRS.0b013e318279a95e
Matsukawa K, Yato Y, Imabayashi H, Hosogane N, Asazuma T, Nemoto K (2015) Biomechanical evaluation of the fixation strength of lumbar pedicle screws using cortical bone trajectory: a finite element study. J Neurosurg Spine 23:471–478. https://doi.org/10.3171/2015.1.Spine141103
Cornaz F, Widmer J, Fasser MR, Snedeker JG, Matsukawa K, Spirig JM, Farshad M (2021) Is a cross-connector beneficial for single level traditional or cortical bone trajectory pedicle screw instrumentation? PLoS One 16:e0253076. https://doi.org/10.1371/journal.pone.0253076
Kim KJ, Kim DH, Lee JI, Choi BK, Han IH, Nam KH (2019) Hounsfield units on lumbar computed tomography for predicting regional bone mineral density. Open Med (Wars) 14:545–551. https://doi.org/10.1515/med-2019-0061
Farshad M, Betz M, Farshad-Amacker NA, Moser M (2017) Accuracy of patient-specific template-guided vs. free-hand fluoroscopically controlled pedicle screw placement in the thoracic and lumbar spine: a randomized cadaveric study. Eur Spine J 26:738–749. https://doi.org/10.1007/s00586-016-4728-5
Matsukawa K, Kaito T, Abe Y (2020) Accuracy of cortical bone trajectory screw placement using patient-specific template guide system. Neurosurg Rev 43:1135–1142. https://doi.org/10.1007/s10143-019-01140-1
Matsukawa K, Yato Y, Imabayashi H, Hosogane N, Abe Y, Asazuma T, Chiba K (2016) Biomechanical evaluation of fixation strength among different sizes of pedicle screws using the cortical bone trajectory: what is the ideal screw size for optimal fixation? Acta Neurochir (Wien) 158:465–471. https://doi.org/10.1007/s00701-016-2705-8
Matsukawa K, Taguchi E, Yato Y, Imabayashi H, Hosogane N, Asazuma T, Nemoto K (2015) Evaluation of the fixation strength of pedicle screws using cortical bone trajectory: what is the ideal trajectory for optimal fixation? Spine 40:E873-878. https://doi.org/10.1097/brs.0000000000000983
Burkhard MD, Cornaz F, Spirig JM, Wanivenhaus F, Loucas R, Fasser MR, Widmer J, Farshad M (2021) Posterior spinal instrumentation and decompression with or without cross-link? N Am Spine Soc J 8:100093. https://doi.org/10.1016/j.xnsj.2021.100093
Cornaz F, Fasser MR, Spirig JM, Snedeker JG, Farshad M, Widmer J (2020) 3D printed clamps improve spine specimen fixation in biomechanical testing. J Biomech 98:109467. https://doi.org/10.1016/j.jbiomech.2019.109467
Cornaz F, Burkhard M, Fasser MR, Spirig JM, Snedeker JG, Farshad M, Widmer J (2021) 3D printed clamps for fixation of spinal segments in biomechanical testing. J Biomech 125:110577. https://doi.org/10.1016/j.jbiomech.2021.110577
Wilke HJ, Wenger K, Claes L (1998) Testing criteria for spinal implants: recommendations for the standardization of in vitro stability testing of spinal implants. Eur Spine J 7:148–154. https://doi.org/10.1007/s005860050045
Cofano F, Marengo N, Ajello M, Penner F, Mammi M, Petrone S, Lavorato A, Zenga F, Garbossa D (2020) The era of cortical bone trajectory screws in spine surgery: a qualitative review with rating of evidence. World Neurosurg 134:14–24. https://doi.org/10.1016/j.wneu.2019.10.079
Makanji H, Schoenfeld AJ, Bhalla A, Bono CM (2018) Critical analysis of trends in lumbar fusion for degenerative disorders revisited: influence of technique on fusion rate and clinical outcomes. Eur Spine J 27:1868–1876. https://doi.org/10.1007/s00586-018-5544-x
Xu M, Yang J, Lieberman I, Haddas R (2019) Stress distribution in vertebral bone and pedicle screw and screw-bone load transfers among various fixation methods for lumbar spine surgical alignment: a finite element study. Med Eng Phys 63:26–32. https://doi.org/10.1016/j.medengphy.2018.10.003
Kim DH, Hwang RW, Lee GH, Joshi R, Baker KC, Arnold P, Sasso R, Park D, Fischgrund J (2020) Comparing rates of early pedicle screw loosening in posterolateral lumbar fusion with and without transforaminal lumbar interbody fusion. Spine J 20:1438–1445. https://doi.org/10.1016/j.spinee.2020.04.021
Arand M, Wilke HJ, Schultheiss M, Hartwig E, Kinzl L, Claes L (2000) Comparative stability of the “internal fixator” and the “universal spine system” and the effect of crosslinking transfixating systems. a biomechanical in vitro study. Biomed Tech (Berl) 45:311–316. https://doi.org/10.1515/bmte.2000.45.11.311
Sakaura H, Ikegami D, Fujimori T, Sugiura T, Mukai Y, Hosono N, Fuji T (2019) Early cephalad adjacent segment degeneration after posterior lumbar interbody fusion: a comparative study between cortical bone trajectory screw fixation and traditional trajectory screw fixation. J Neurosurg Spine 32:155–159. https://doi.org/10.3171/2019.8.Spine19631
Sakaura H, Miwa T, Yamashita T, Kuroda Y, Ohwada T (2018) Cortical bone trajectory screw fixation versus traditional pedicle screw fixation for 2-level posterior lumbar interbody fusion: comparison of surgical outcomes for 2-level degenerative lumbar spondylolisthesis. J Neurosurg Spine 28:57–62. https://doi.org/10.3171/2017.5.Spine161154
Lee GW, Ahn MW (2018) Comparative study of cortical bone trajectory-pedicle screw (cortical screw) versus conventional pedicle screw in single-level posterior lumbar interbody fusion: a 2-year post hoc analysis from prospectively randomized data. World Neurosurg 109:e194–e202. https://doi.org/10.1016/j.wneu.2017.09.137
Widmer J, Cornaz F, Scheibler G, Spirig JM, Snedeker JG, Farshad M (2020) Biomechanical contribution of spinal structures to stability of the lumbar spine-novel biomechanical insights. Spine J 20:1705–1716. https://doi.org/10.1016/j.spinee.2020.05.541
Crawford NR, Peles JD, Dickman CA (1998) The spinal lax zone and neutral zone: measurement techniques and parameter comparisons. J Spinal Disord 11:416–429
Acknowledgements
The authors gratefully thank Mauro Suter for his technical support during this study. The authors further thank Medacta International (Castel San Peitro, Switzerland) for providing the implants used for this study. Imaging was performed with support of the Swiss Center for Musculoskeletal Imaging, SCMI, Balgrist Campus AG, Zurich.
Funding
Open access funding provided by University of Zurich.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors state that they have no potential conflict of interest in relation to this study's content. MF is consultant for Medacta, but unrelated to the content of this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Burkhard, M.D., Cornaz, F., Spirig, J.M. et al. Residual motion of cortical versus pedicle screw constructs after decompression, interbody fusion and cross-link augmentation. Eur Spine J 32, 1401–1410 (2023). https://doi.org/10.1007/s00586-023-07596-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-023-07596-6