Abstract
Purpose
To evaluate proximal junctional biomechanics of a MLSS relative to traditional pedicle screw fixation at the proximal extent of T10-pelvis posterior instrumentation constructs (T10-p PSF).
Methods
A previously validated three-dimensional osseoligamentous spinopelvic finite element (FE) model was used to compare proximal junctional range-of-motion (ROM), vertebral body stresses, and discal biomechanics between two groups: (1) T10-p with a T10-11 MLSS (“T10-11 MLSS”) and (2) T10-p with a traditional T10 pedicle screw (“Traditional T10-PS”).
Results
The T10-11 MLSS had a 5% decrease in T9 cortical bone stress compared to Traditional T10-PS. Conversely, the T10 and T11 bone stresses increased by 46% and 98%, respectively, with T10-11 MLSS compared to Traditional T10-PS. Annular stresses and intradiscal pressures (IDP) were similar at T9-T10 between constructs. At the T10-11 disc, T10-11 MLSS decreased annular stresses by 29% and IDP by 48% compared to Traditional T10-PS. Adjacent ROM (T8-9 & T9-10) were similar between T10-11 MLSS and Traditional T10-PS. T10-11 MLSS had 39% greater ROM at T10-11 and 23% less ROM at T11-12 compared to Traditional T10-PS.
Conclusions
In this FE analysis, a T10-11 MLSS at the proximal extent of T10-pelvis posterior instrumentation resulted in increased T10 and T11 cortical bone stresses, decreased discal annular stress and IDP and increased ROM at T10-11, and no change in ROM at the adjacent level. Given the complex and multifactorial nature of proximal junctional kyphosis, these results require additional biomechanical and clinical evaluations to determine the clinical utility of MLSS on the proximal junctions of thoracolumbar posterior instrumented fusions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Proximal junctional kyphosis (PJK) and fractures (PJF) are common causes of revision operations that jeopardize patient quality of life, improvement in clinical outcomes, cost-effectiveness, and utility of index operations for adult spinal deformity (ASD) [1,2,3]. Two failure mechanisms constitute PJK/PJF: ligamentous failure and osseous failure [4]. Ligamentous failure often is due to iatrogenic alteration of the posterior supraspinous and interspinous ligaments, spinous process, and paraspinal musculature and can result in catastrophic subluxation at the proximal junction [4,5,6,7,8,9]. Osseous failure resulting in PJK/PJF presents with a fracture of the upper instrumented vertebrae (UIV) and/or fracture of the supra-adjacent vertebrae (UIV + 1) [4]. Fracture patterns are heterogeneous, ranging from a compression fracture with mild kyphosis, severe UIV collapse, or fracture-dislocation of the UIV + 1 [4]. Translation instability is often clinically significant and commonly results in myelopathy necessitating revision surgery [9].
Minimizing the risk of developing PJK/PJF is complex and multifactorial, including creating a smooth load transfer from the instrumented levels to the un-instrumented at the proximal segments, restoring harmonious sagittal alignment, and minimizing soft tissue disruption at the proximal junction. While multiple techniques have been proposed to achieve this goal, one that is relatively unexplored is a screw placed into the UIV (i.e., T10) through the UIV/UIV-1 disc space (T10-T11) of a T10-pelvis posterior instrumentation construct. This type of thoracic trans-discal screw has been termed a “Multi-Level Stabilization Screw (MLSS)” [10,11,12,13,14]. The purpose of this study is to compare the proximal junctional biomechanics of T10 to pelvis posterior instrumented constructs supplemented with a T10-11 MLSS to traditional pedicle screw fixation at T10.
Materials and methods
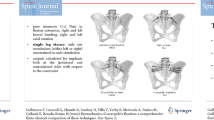
A previously validated three-dimensional osseoligamentous spinopelvic finite element model (T10-pelvis) was used (Fig. 1) [15, 16]. The initial intact model of the ligamentous spine was reconstructed from computed tomography (CT) scans of a human spine using MIMICS (Materialize Inc., Leuven, Belgium) software. The IAFE-MESH (University of Iowa, Iowa) and HyperMesh (Altair Engineering, Michigan, USA) software were used to create hexahedral elements (C3D8) of the vertebrae and tetrahedral elements (C3D4) of the pelvis. The meshed components were assembled in the Abaqus 6.14 (DassaultSystemes, Simulia Inc., Providence, RI, USA) software. The spinal and sacroiliac ligaments were modeled using truss elements. In the vertebral body, a layer of 0.5 mm cortical bone was simulated to surround the cancellous bone.
The material properties for cortical/cancellous bones, annulus, nucleus, ligaments, and joints were selected based on the literature and assigned (Table 1). [17, 24] Isotropic material properties were utilized for bone due to the relatively uniform structure and response to stress in all directions, which is characteristic of bone tissue. The intervertebral discs were composed of annulus fibrosis and nucleus pulposus. To accurately capture the complex behavior of these two components, hyperelastic anisotropic material models were adopted from the literature and employed [17, 24]. The annulus fibrosis was simulated using a solid ground substance (C3D8 elements) that was reinforced with rebar elements (embedded into the ground matrix with ± 30° angles) [17, 24]. The nucleus pulposus was modeled using C3D8 elements with hyper-elastic Mooney-Rivlin formulation. Articular surfaces (sacroiliac joints, spine facets, articular cartilages, and pubic symphysis) were assigned an exponential contact that adjusts the force as the distance between the surfaces decrease [17, 24]. The cartilaginous layer at the sacroiliac joint was simulated using soft contact with force-adjusting exponential behavior [17, 24]. Hypoelastic nonlinear material behavior was defined for each spinal and pelvis ligaments based on their force–deflection curve (Table 1).
Models
Two construct models were developed: (1) “Traditional T10-PS”: instrumented with pedicle screws and rods from T10-pelvis and (2) “T10-11 MLSS”: instrumented with pedicle screws and rods from T10-pelvis with the UIV screw placed from T11 into T10 through the T10-T11 disc (Fig. 1). All instrumentation was designed in SolidWorks (Dassault Systems, SolidWorks Corporation, Waltham, MA, USA) software and imported into Abaqus for model development. Each pedicle screw was modeled in a two-part (including a tulip & a shaft) connected with a ball & socket joint. All screws and rods were titanium alloy. Rod diameters were 5.5 mm.
A two-step analysis was performed. In step one, the spine model was pre-loaded with axial compression load to simulate body weight using the following load technique: 300 N to the thoracic spine, 400 N to the lumbar spine, and 400 N to the sacrum [17, 18]. In step two, pure moments of 7.5 Nm were applied to the top endplate of the T10 vertebra in all three anatomical directions. In both steps, the acetabulum surfaces of the pelvis were fixed in all degrees of freedom.
Outcome measures
For each instrumentation technique, the range of motion (ROM), maximum cortical stresses, intervertebral disc annular stresses, and intervertebral disc pressures (IDP) were evaluated and compared between Traditional T10-PS constructs and T10-11 MLSS constructs.
Results
Vertebral body stresses (Fig. 2)
Stresses at the cortical bone of T9 decreased by 5% in the T10-11 MLSS construct compared to Traditional T10-PS. Conversely, the stresses within the cortical bones of T10 and T11 increased by 46% and 98%, respectively, with the T10-11 MLSS compared to Traditional T10-PS.
Discal biomechanics (Figs. 3 and 4)
Annular stresses were similar at the T9-T10 disc between the T10-11 MLSS construct and the Traditional T10-PS construct. Intradiscal pressures were also similar at the T9-T10 disc between T10-11 MLSS and Traditional T10-PS. At the T10-11 disc, T10-11 MLSS was found to decrease annular stresses by 29% and IDP by 48% compared to Traditional T10-PS.
Range of motion (Fig. 5)
Range of motion at the T8-9 and T9-10 vertebrae were similar (within 1%) between T10-11 MLSS and Traditional T10-PS. The T10-11 MLSS constructs had 39% greater ROM in the T10-11 segment and 23% less ROM in the T11-12 segment compared to Traditional T10-PS.
Discussion
In this study, we present for the first time a finite element analysis of proximal junctional biomechanics of a screw placed into the UIV (T10) through the T10-T11 disc at the proximal extent of T10-pelvis posterior instrumentation construct [“Multi-Level Stabilization Screw (MLSS)]” compared to traditional pedicle screw fixation at T10. Our results demonstrated biomechanical differences at the proximal junction between the two constructs in regard to bone stresses, disc parameters, and ROM. Specifically, the T10-T11 MLSS was found to result in slightly lower bone stress at the UIV + 1 (T9) but significantly greater stresses in the UIV (T10; + 46%) and UIV-1 (T11; + 98%) compared to conventional T10 pedicle screw fixation. No differences in adjacent segment discal parameters (T9-10) were found, though annular stresses and IDP decreased notably in the T10-11 disc through which the T10-11 MLSS traversed. Furthermore, ROM at T10-11 increased in the intradiscal screw model by 39% compared to the traditional construct, while ROMs cranially to the UIV (T8-9 and T9-10) were similar between the two models.
The concept of a MLSS was initially described for stabilization of the lumbosacral junction in moderate- to high-grade spondylolisthesis with resultant high fusion rates, low incidence of neurologic complications, decreased operative duration, and more rapid return to activity [19,20,21]. In a cadaveric biomechanical study at the lumbosacral junction, Minamide et al. reported 1.6–1.8 times stiffer fixation with transdiscal screws compared to pedicle screw fixation and had no difference in stiffness compared to combined interbody/pedicle screw fixation [22]. In a comparative case–control study assessing outcomes of transdiscal screw versus pedicle screw fixation for high-grade L5-S1 isthmic spondylolisthesis, improved functional and radiographic outcomes at 2-year follow-up were noted in the transdiscal screw cohort [23]. Given this documented safety and efficacy for lumbosacral pathology as well as the perceived advantage that transdiscal pedicle screws increase the stability of a construct as they purchase multiple cortical layers of two vertebrae, the use of MLSS expanded to fixation of thoracic spine pathology.
Documented clinical use of MLSS in the thoracic spine is limited but encouraging. In 2013, Nottmeier et al. presented the first case report of 12 patients undergoing cervicothoracic or thoracic fusions in whom MLSS screws were placed using navigation to achieve improved purchase in the setting of osteoporosis, failed pedicle screws, and/or at the proximal extent of the constructs [13]. Use of MLSS has also been reported to be achieved safely via percutaneous techniques in patients with diffuse idiopathic skeletal hyperostosis, and were favored over traditional pedicle screw fixation given a significant reduction in screw loosening and 134% higher insertional torque [10]. The implementation of MLSS in adult deformity surgery at the UIV was first reported in a case series of 20 patients by Sandquist et al. who found a 0% incidence of PJK and PJF [7]. In turn, it was concluded that MLSS is safe and efficacious in the thoracic spine to reduce the incidence of proximal junctional complications [7]. Our study augments this previously published clinical study through a finite element analysis and provides greater insight into the biomechanics of this specific technique at the proximal junction of the long posterior thoracolumbar junction.
Our findings, in sum, suggest that the T10-T11 MLSS increases the range of motion and lowers intradiscal parameters at the T10-11 segment (relative to traditional pedicle screw fixation), which in turn transitions to an unfused segment. Our ROM findings are consistent with the only other prior biomechanical analysis of thoracic MLSS by Rodriguez-Martinez et al. [14]. That there is increased ROM at T10-11, no differences in T9-10 ROM, and considerably increased stresses in the T10 and T11 vertebral bodies in the setting of the T10-11 MLSS may suggest an increased risk of osseous failure/fractures of the UIV. However, that there were no muscle forces simulated may potentially underestimate the biomechanical benefits of the MLSS technique given that the MLSS screw may be less disruptive to the proximal junction’s soft tissues, as its relatively inferior and medial start point allows for preservation of the soft tissue attachments to the lateral aspect of the UIV’s transverse processes. Comparatively, the more cranial and lateral start site of a UIV pedicle screw may be more destabilizing to the soft tissue posterior tension band, which is a known risk factor for developing PJK [5,6,7,8].
While the ultimate clinical behavior of the MLSS remains incompletely understood, it is reassuring that the aforementioned cohort presented by Sandquist et al. did not result in an unacceptably high rate of PJK/PJF [7]. Although Sandquist et al. did not report any complications associated with the MLSS technique, some potential downsides of the MLSS may include a breach of the UIV’s cranial endplate that may result in accelerated adjacent segment disease, limited fixation of the MLSS in the UIV as a result of a less inclined caudal-cranial trajectory, challenges placing the MLSS screw in the appropriate position by a free-hand technique, increased risk of pseudarthrosis as a result of less rigid fixation across the T10-11 segment, and possibly higher risk of screw pull-out given the cranially directed nature of the screw trajectory [25]. Note these risks are postulations, as they have not been reported in the literature given limited clinical experiences with this technique. Furthermore, additional short- and long-term clinical challenges may be associated with the MLSS technique to which we are not privy. As this rarely used technique may have more potential risks than benefits, additional clinical series would be helpful to ultimately determine if stabilization of the proximal segments of long thoracolumbar fusions with MLSS is safe, effective, and durable.
The results of this study should be considered in the context of its limitations. While we believe the accuracy of this finite element analysis model is acceptable given its use of a well-established, previously validated model, there are several factors that may jeopardize its accuracy in simulating the forces following a long posterior thoracolumbar instrumented fusion. These include simulations performed using uncomplicated geometries of the implants and simplified contacts and constraints as well as no muscle forces. An additional limitation is that proximal junctional biomechanics may be influenced by other factors, including, but not limited to, rod diameter, rod material, sagittal alignment, and bone quality. Moreover, as a sensitivity analysis is the process of evaluating how the output of FE models changes with respect to variations in the input parameters (i.e. material properties, boundary conditions, geometry, mesh size, loads), our model, in turn, may not be generalizable to all clinical scenarios in which different materials are used for different instrumentation strategies (i.e. cobalt chrome rod, titanium rod, pedicle screw with cobalt chrome tulip head and titanium shaft, all titanium pedicle screw, rod sizes 5.5 vs. 6.0 vs. 6.25 mm, etc.). However, it should be noted that while the model has these limitations, the use of comparative analyses (relative to the “Traditional T10-PS” construct) makes our reported relative differences of greater credence than individual absolute values. While we report relative differences between the different screw configurations, we are unable to comment upon the biomechanical and clinical significances of our observed biomechanical differences and relative long-term clinical performance of the different instrumentation configurations evaluated in this study, particularly because the exact margin of error, as well as the margin of important difference, are not known. Despite our model not being able to capture every nuanced difference in instrumentation strategy used clinically, we do feel that there is potential clinical applicability of our data. However, the ultimate determination of the clinical applicability of our findings will require additional clinical investigations. Ideally, the result of this study may be considered a unique addition to the growing literature on a unique fixation strategy (MLSS) at the proximal junction of long posterior instrumented fusions that will stimulate further discussion and inquiry into their clinical utility.
Conclusions
In this finite element analysis, a screw placed into the UIV (T10) through the T10-T11 disc at the proximal extent of T10-pelvis posterior instrumentation construct resulted in increased cortical bone stresses at T10 and T11, decreased discal parameters (annular stresses and IDP) and increased ROM at T10-11, and no change in ROM at the adjacent level (T9-10). Given the complex and multifactorial nature of PJK/PJF, these results require additional biomechanical evaluations as well as clinical investigations so as to determine clinical utility and in vivo effects of MLSS on the proximal junctions of long thoracolumbar posterior instrumented fusions.
Data availability
Data is available on request from the authors.
References
Riley MS, Bridwell KH, Lenke LG, Dalton J, Kelly MP (2018) Health-related quality of life outcomes in complex adult spinal deformity surgery. J Neurosurg Spine 28(2):194–200
Durand WM, Babu JM, Hamilton DK et al (2022) Adult spinal deformity surgery is associated with increased productivity and decreased absenteeism from work and school. Spine 47(4):287–294
Ryu WHA, Cheong M, Platt A et al (2021) Patient Satisfaction Following Minimally Invasive and Open Surgeries for Adult Spinal Deformity. World Neurosurg 155:e301–e314
Yagi M, King AB, Boachie-Adjei O (2012) Incidence, risk factors, and natural course of proximal junctional kyphosis: surgical outcomes review of adult idiopathic scoliosis. Minimum 5 years of follow-up. Spine 37(17):1479–1489
Gupta MC, Wijesekera S, Sossan A et al (2007) Reliability of radiographic parameters in neuromuscular scoliosis. Spine 32(6):691–695
Ha Y, Maruo K, Racine L et al (2013) Proximal junctional kyphosis and clinical outcomes in adult spinal deformity surgery with fusion from the thoracic spine to the sacrum: a comparison of proximal and distal upper instrumented vertebrae. J Neurosurg Spine 19(3):360–369
Sandquist L, Carr D, Tong D, Gonda R, Soo TM (2015) Preventing proximal junctional failure in long segmental instrumented cases of adult degenerative scoliosis using a multilevel stabilization screw technique. Surg Neurol Int 6:112
Vercoulen TFG, Doodkorte RJP, Roth A, de Bie R, Willems PC (2022) Instrumentation techniques to prevent proximal junctional kyphosis and proximal junctional failure in adult spinal deformity correction: a systematic review of clinical studies. Global Spine J 12(6):1282–1296
Fakhre E, Kelly MJ, Mo FF (2022) Proximal junctional kyphosis. Semin Spine Surg 34(1):100926
Takeuchi T, Hosogane N, Yamagishi K, Satomi K, Matsukawa K, Ichimura S (2020) Results of using a novel percutaneous pedicle screw technique for patients with diffuse idiopathic skeletal hyperostosis-the single or double endplates penetrating screw (SEPS/DEPS) technique. Spine Surg Relat Res 4(3):261–268
Filis AK, Aghayev K, Schaller B, Luksza J, Vrionis FD (2016) Transdiscal mid- and upper thoracic vertebroplasty: first description of 2 exemplary cases. J Neurosurg Spine 25(2):193–197
Mehdizade A, Payer M, Somon T et al (2004) Percutaneous vertebroplasty through a transdiscal access route after lumbar transpedicular instrumentation. Spine J 4(4):475–479
Nottmeier EW, Pirris SM (2013) Placement of thoracic transvertebral pedicle screws using 3D image guidance. J Neurosurg Spine 18(5):479–483
Rodriguez-Martinez NG, Savardekar A, Nottmeier EW et al (2016) Biomechanics of transvertebral screw fixation in the thoracic spine: an in vitro study. J Neurosurg Spine 25(2):187–192
Goel VK, Grauer JN, Patel TC et al (2005) Effects of charité artificial disc on the implanted and adjacent spinal segments mechanics using a hybrid testing protocol. Spine 30(24):2755–2764
Goel VK, Mehta A, Jangra J et al (2007) Anatomic facet replacement system (AFRS) restoration of lumbar segment mechanics to intact: a finite element study and in vitro cadaver investigation. SAS J 1(1):46–54
Seyed Vosoughi A, Joukar A, Kiapour A et al (2019) Optimal satellite rod constructs to mitigate rod failure following pedicle subtraction osteotomy (PSO): a finite element study. Spine J 19(5):931–941
Patwardhan AG, Havey RM, Carandang G et al (2003) Effect of compressive follower preload on the flexion-extension response of the human lumbar spine. J Orthop Res 21(3):540–546
Abdu WA, Wilber RG, Emery SE (1994) Pedicular transvertebral screw fixation of the lumbosacral spine in spondylolisthesis. A new technique for stabilization. Spine 19(6):710–715
Logroscino CA, Tamburrelli FC, Scaramuzzo L, Schirò GR, Sessa S, Proietti L (2012) Transdiscal L5–S1 screws for the treatment of adult spondylolisthesis. Eur Spine J 21(Suppl 1):S128–S133
Rindler RS, Miller BA, Eshraghi SR et al (2016) Efficacy of transsacral instrumentation for high-grade spondylolisthesis at L5–S1: a systematic review of the literature. World Neurosurg 95:623.e11-623.e19
Minamide A, Akamaru T, Yoon ST, Tamaki T, Rhee JM, Hutton WC (2003) Transdiscal L5–S1 screws for the fixation of isthmic spondylolisthesis: a biomechanical evaluation. J Spinal Disord Tech 16(2):144–149
Collados-Maestre I, Lizaur-Utrilla A, Bas-Hermida T, Pastor-Fernandez E, Gil-Guillen V (2016) Transdiscal screw versus pedicle screw fixation for high-grade L5–S1 isthmic spondylolisthesis in patients younger than 60 years: a case-control study. Eur Spine J 25(6):1806–1812
Lindsey D, Kiapour A, Yerby S, Goel V (2015) Sacroiliac joint fusion minimally affects adjacent lumbar segment motion: a finite element study. Int J Spine Surg 9:64
Harris AB, Kebaish FN, Puvanesarajah V et al (2021) Caudally directed upper-instrumented vertebra pedicle screws associated with minimized risk of proximal junctional failure in patients with long posterior spinal fusion for adult spinal deformity. Spine J 21(7):1072–1079
Funding
No funding was received for this work.
Author information
Authors and Affiliations
Contributions
Collins, Shah, Shekouhi, Goel, Theologis: Made substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data; or the creation of new software used in the work. Drafted the work or revised it critically for important intellectual content. Approved the version to be published. Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Conflict of interest
None relevant to the submitted work.
IRB approval
Not applicable.
Informed consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Collins, A.P., Shah, A.A., Shekouhi, N. et al. Biomechanical analysis of a trans-discal, multi-level stabilization screw (MLSS) at the upper instrumented vertebra (UIV) of long posterior thoracolumbar instrumentations. Spine Deform 12, 953–959 (2024). https://doi.org/10.1007/s43390-024-00862-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43390-024-00862-7