Abstract
Purpose
To investigate the interaction of telomerase activity and telomere length on neuro-protection or neuro-degeneration effects after spinal cord injury (SCI).
Methods
A contusive SCI model was developed using 56 Sprague–Dawley rats. Seven rats were allocated into acute injury phase groups (1, 3, 8, 24, and 48 h), and sub-acute and chronic injury phase groups (1, 2, and 4 weeks). Telomerase activity was assessed by telomerase reverse transcriptase (TERT) and telomeric repeat binding factor-2 (TERF-2). Differentiation of activated neural stem cells was investigated by co-expression of neuronal/glial cell markers. Apoptosis expression was also investigated by caspase-3, 8, and 9 using terminal deoxynucleotidyl transferase dUTP nick end labelling staining. Immunofluorescence staining and western blotting were performed for quantitative analyses.
Results
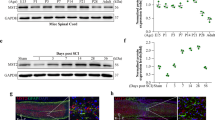
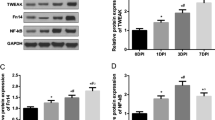
Expression of TERT increased gradually until 24 h post-injury, and was decreased following SCI (P < 0.05). TERF-2 also was increased following SCI until 24 h post-injury and then decreased with time (P < 0.05). Co-localization of TERT and TERF-2 was higher at 24 h post-injury. High expression of TERT was seen in neurons (Neu N Ab), however, expression of TERT was relatively lower in astrocytes and oligodendrocytes. Apoptosis analysis showed persistent high expression of caspases-3, -9, and -8 during the observation period.
Conclusions
Increased TERT and TERF-2 activity were noted 24 h post-injury in the acute phase of SCI with TERF-2 maintaining telomeric-repeat length. Our results suggest that increased activity of telomere maintenance may be related to neuro-protective mechanisms against subsequent apoptosis resulting from DNA damage after acute SCI.







Similar content being viewed by others
References
Seo JY, Kim YH, Kim JW, Kim SI, Ha KY (2015) Effects of therapeutic hypothermia on apoptosis and autophagy after spinal cord injury in rats. Spine (Phila Pa 1976) 40:883–890. https://doi.org/10.1097/brs.0000000000000845
Ha KY, Kim YH (2008) Neuroprotective effect of moderate epidural hypothermia after spinal cord injury in rats. Spine (Phila Pa 1976) 33:2059–2065. https://doi.org/10.1097/BRS.0b013e31818018f6
Multani AS, Ozen M, Narayan S, Kumar V, Chandra J, McConkey DJ, Newman RA, Pathak S (2000) Caspase-dependent apoptosis induced by telomere cleavage and TRF2 loss. Neoplasia 2:339–345. https://doi.org/10.1038/sj.neo.7900105
Niu C, Yip HK (2011) Neuroprotective signaling mechanisms of telomerase are regulated by brain-derived neurotrophic factor in rat spinal cord motor neurons. J Neuropathol Exp Neurol 70:634–652. https://doi.org/10.1097/NEN.0b013e318222b97b
Tao X, Ming-Kun Y, Wei-Bin S, Hai-Long G, Rui K, Lai-Yong T (2013) Role of telomerase reverse transcriptase in glial scar formation after spinal cord injury in rats. Neurochem Res 38:1914–1920. https://doi.org/10.1007/s11064-013-1097-x
Turner KJ, Vasu V, Griffin DK (2019) Telomere biology and human phenotype. Cells 8:73. https://doi.org/10.3390/cells8010073
Mukherjee AK, Sharma S, Sengupta S, Saha D, Kumar P, Hussain T, Srivastava V, Roy SD, Shay JW, Chowdhury S (2018) Telomere length-dependent transcription and epigenetic modifications in promoters remote from telomere ends. PLoS Genet 14:e1007782. https://doi.org/10.1371/journal.pgen.1007782
Ding D, Zhou J, Wang M, Cong YS (2013) Implications of telomere-independent activities of telomerase reverse transcriptase in human cancer. FEBS J 280:3205–3211. https://doi.org/10.1111/febs.12258
Kovalenko OA, Caron MJ, Ulema P, Medrano C, Thomas AP, Kimura M, Bonini MG, Herbig U, Santos JH (2010) A mutant telomerase defective in nuclear-cytoplasmic shuttling fails to immortalize cells and is associated with mitochondrial dysfunction. Aging Cell 9:203–219. https://doi.org/10.1111/j.1474-9726.2010.00551.x
Park JI, Venteicher AS, Hong JY, Choi J, Jun S, Shkreli M, Chang W, Meng Z, Cheung P, Ji H, McLaughlin M, Veenstra TD, Nusse R, McCrea PD, Artandi SE (2009) Telomerase modulates Wnt signalling by association with target gene chromatin. Nature 460:66–72. https://doi.org/10.1038/nature08137
Sahin E, Colla S, Liesa M, Moslehi J, Müller FL, Guo M, Cooper M, Kotton D, Fabian AJ, Walkey C, Maser RS, Tonon G, Foerster F, Xiong R, Wang YA, Shukla SA, Jaskelioff M, Martin ES, Heffernan TP, Protopopov A, Ivanova E, Mahoney JE, Kost-Alimova M, Perry SR, Bronson R, Liao R, Mulligan R, Shirihai OS, Chin L, DePinho RA (2011) Telomere dysfunction induces metabolic and mitochondrial compromise. Nature 470:359–365. https://doi.org/10.1038/nature09787
Singhapol C, Pal D, Czapiewski R, Porika M, Nelson G, Saretzki GC (2013) Mitochondrial telomerase protects cancer cells from nuclear DNA damage and apoptosis. PLoS One 8:e52989. https://doi.org/10.1371/journal.pone.0052989
Cicconi A, Chang S (2020) Shelterin and the replisome: at the intersection of telomere repair and replication. Curr Opin Genet Dev 60:77–84. https://doi.org/10.1016/j.gde.2020.02.016
La Torre D, Conti A, Aguennouz MH, De Pasquale MG, Romeo S, Angileri FF, Cardali S, Tomasello C, Alafaci C, Germanò A (2013) Telomere length modulation in human astroglial brain tumors. PLoS One 8:e64296. https://doi.org/10.1371/journal.pone.0064296
Grammatikakis I, Zhang P, Mattson MP, Gorospe M (2016) The long and the short of TRF2 in neurogenesis. Cell Cycle 15:3026–3032. https://doi.org/10.1080/15384101.2016.1222339
de Lange T (2005) Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev 19:2100–2110. https://doi.org/10.1101/gad.1346005
Rai R, Chen Y, Lei M, Chang S (2016) TRF2-RAP1 is required to protect telomeres from engaging in homologous recombination-mediated deletions and fusions. Nat Commun 7:10881. https://doi.org/10.1038/ncomms10881
Emery E, Aldana P, Bunge MB, Puckett W, Srinivasan A, Keane RW, Bethea J, Levi AD (1998) Apoptosis after traumatic human spinal cord injury. J Neurosurg 89:911–920. https://doi.org/10.3171/jns.1998.89.6.0911
Li GL, Brodin G, Farooque M, Funa K, Holtz A, Wang WL, Olsson Y (1996) Apoptosis and expression of Bcl-2 after compression trauma to rat spinal cord. J Neuropathol Exp Neurol 55:280–289. https://doi.org/10.1097/00005072-199603000-00003
Lee JS, Jeong SW, Cho SW, Juhn JP, Kim KW (2015) Relationship between initial telomere length, initial telomerase activity, age, and replicative capacity of nucleus pulposus chondrocytes in human intervertebral discs: what is a predictor of replicative potential? PLoS One 10:e0144177. https://doi.org/10.1371/journal.pone.0144177
Cheng A, Shin-ya K, Wan R, Tang SC, Miura T, Tang H, Khatri R, Gleichman M, Ouyang X, Liu D, Park HR, Chiang JY, Mattson MP (2007) Telomere protection mechanisms change during neurogenesis and neuronal maturation: newly generated neurons are hypersensitive to telomere and DNA damage. J Neurosci 27:3722–3733. https://doi.org/10.1523/jneurosci.0590-07.2007
González-Giraldo Y, Forero DA, Echeverria V, Gonzalez J, Ávila-Rodriguez M, Garcia-Segura LM, Barreto GE (2016) Neuroprotective effects of the catalytic subunit of telomerase: a potential therapeutic target in the central nervous system. Ageing Res Rev 28:37–45. https://doi.org/10.1016/j.arr.2016.04.004
Sobrido-Cameán D, Barreiro-Iglesias A (2018) Role of caspase-8 and fas in cell death after spinal cord injury. Front Mol Neurosci 11:101. https://doi.org/10.3389/fnmol.2018.00101
Denchi EL (2009) Give me a break: how telomeres suppress the DNA damage response. DNA Repair (Amst) 8:1118–1126. https://doi.org/10.1016/j.dnarep.2009.04.013
Lobanova A, She R, Pieraut S, Clapp C, Maximov A, Denchi EL (2017) Different requirements of functional telomeres in neural stem cells and terminally differentiated neurons. Genes Dev 31:639–647. https://doi.org/10.1101/gad.295402.116
Kim JW, Ha KY, Molon JN, Kim YH (2013) Bone marrow-derived mesenchymal stem cell transplantation for chronic spinal cord injury in rats: comparative study between intralesional and intravenous transplantation. Spine (Phila Pa 1976) 38:E1065-1074. https://doi.org/10.1097/BRS.0b013e31829839fa
Lee JY, Ha KY, Kim JW, Seo JY, Kim YH (2014) Does extracorporeal shock wave introduce alteration of microenvironment in cell therapy for chronic spinal cord injury? Spine (Phila Pa 1976) 39:E1553-1559. https://doi.org/10.1097/brs.0000000000000626
Klapper W, Shin T, Mattson MP (2001) Differential regulation of telomerase activity and TERT expression during brain development in mice. J Neurosci Res 64:252–260. https://doi.org/10.1002/jnr.1073
Fu W, Killen M, Culmsee C, Dhar S, Pandita TK, Mattson MP (2000) The catalytic subunit of telomerase is expressed in developing brain neurons and serves a cell survival-promoting function. J Mol Neurosci 14:3–15. https://doi.org/10.1385/jmn:14:1-2:003
Li J, Qu Y, Chen D, Zhang L, Zhao F, Luo L, Pan L, Hua J, Mu D (2013) The neuroprotective role and mechanisms of TERT in neurons with oxygen–glucose deprivation. Neuroscience 252:346–358. https://doi.org/10.1016/j.neuroscience.2013.08.015
Spilsbury A, Miwa S, Attems J, Saretzki G (2015) The role of telomerase protein TERT in Alzheimer’s disease and in tau-related pathology in vitro. J Neurosci 35:1659–1674. https://doi.org/10.1523/jneurosci.2925-14.2015
Ruis P, Van Ly D, Borel V, Kafer GR, McCarthy A, Howell S, Blassberg R, Snijders AP, Briscoe J, Niakan KK, Marzec P, Cesare AJ, Boulton SJ (2021) TRF2-independent chromosome end protection during pluripotency. Nature 589:103–109. https://doi.org/10.1038/s41586-020-2960-y
Okamoto K, Bartocci C, Ouzounov I, Diedrich JK, Yates JR 3rd, Denchi EL (2013) A two-step mechanism for TRF2-mediated chromosome-end protection. Nature 494:502–505. https://doi.org/10.1038/nature11873
Sarek G, Vannier JB, Panier S, Petrini JHJ, Boulton SJ (2015) TRF2 recruits RTEL1 to telomeres in S phase to promote t-loop unwinding. Mol Cell 57:622–635. https://doi.org/10.1016/j.molcel.2014.12.024
Feuerhahn S, Chen LY, Luke B, Porro A (2015) No DDRama at chromosome ends: TRF2 takes centre stage. Trends Biochem Sci 40:275–285. https://doi.org/10.1016/j.tibs.2015.03.003
Benarroch-Popivker D, Pisano S, Mendez-Bermudez A, Lototska L, Kaur P, Bauwens S, Djerbi N, Latrick CM, Fraisier V, Pei B, Gay A, Jaune E, Foucher K, Cherfils-Vicini J, Aeby E, Miron S, Londoño-Vallejo A, Ye J, Le Du MH, Wang H, Gilson E, Giraud-Panis MJ (2016) TRF2-mediated control of telomere DNA topology as a mechanism for chromosome-end protection. Mol Cell 61:274–286. https://doi.org/10.1016/j.molcel.2015.12.009
Zhao F, Qu Y, Xiong T, Duan Z, Ye Q, Mu D (2012) The neuroprotective role of TERT via an antiapoptotic mechanism in neonatal rats after hypoxia-ischemia brain injury. Neurosci Lett 515:39–43. https://doi.org/10.1016/j.neulet.2012.03.014
Hall ED, Yonkers PA, Andrus PK, Cox JW, Anderson DK (1992) Biochemistry and pharmacology of lipid antioxidants in acute brain and spinal cord injury. J Neurotrauma 9(Suppl 2):S425-442
Funding
This research was supported by the 2017–2018 Korea Medical Institute Research Grant.
Author information
Authors and Affiliations
Contributions
Conceptualization, D.G.C., J.W.K, and K.Y.H.; Methodology, D.G.C., and J.W.K.; Validation, J.W.K., and H.J.K.; Formal analysis, J.W.K. and H.J.K.; Investigation, D.G.C, J.W.K, H.J.K, and K.Y.H.; Writing—Original Draft, D.G.C, and J.W.K.; Writing—Review & Editing, H.J.K., Y.H.K., S.I.K. and K.Y.H.; Funding Acquisition, H.K.Y.; Resources, D.G.C., J.W.K, and K.Y.H.; Supervision, K.Y.H.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
All procedures involving animals were in compliance with the Laboratory Animal Welfare Act and the Guidelines and Policies for Rodent Survival Surgery, as required by the Animal Studies Committee of the Catholic University of Korea (IACUC Approval No. 2014-0192-02).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chang, DG., Kim, JW., Kim, H.J. et al. The neuro-protective role of telomerase via TERT/TERF-2 in the acute phase of spinal cord injury. Eur Spine J 32, 2431–2440 (2023). https://doi.org/10.1007/s00586-023-07561-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-023-07561-3