Abstract
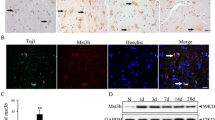
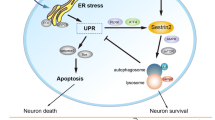
Spinal cord injury (SCI) constitutes a significant clinical challenge, and there is extensive research focused on identifying molecular activities that can facilitate the repair of spinal cord injuries. Mammalian sterile 20-like kinase 2 (MST2), a core component of the Hippo signaling pathway, plays a key role in apoptosis and cell growth. However, its role in neurite outgrowth after spinal cord injury remains unknown. Through comprehensive in vitro and in vivo experiments, we demonstrated that MST2, predominantly expressed in neurons, actively participated in the natural development of the CNS. Post-SCI, MST2 expression significantly increased, indicating its activation and potential role in the early stages of neural recovery. Detailed analyses showed that MST2 knockdown impaired neurite outgrowth and motor function recovery, whereas MST2 overexpression led to the opposite effects, underscoring MST2’s neuroprotective role in enhancing neural repair. Further, we elucidated the mechanism underlying MST2’s action, revealing its interaction with AKT and positive regulation of AKT activity, a well-established promoter of neurite outgrowth. Notably, MST2’s promotion of neurite outgrowth and motor functional recovery was diminished by AKT inhibitors, highlighting the dependency of MST2’s neuroprotective effects on AKT signaling. In conclusion, our findings affirmed MST2’s pivotal role in fostering neuronal neurite outgrowth and facilitating functional recovery after SCI, mediated through its positive modulation of AKT activity. In conclusion, our findings confirmed MST2’s crucial role in neural protection, promoting neurite outgrowth and functional recovery after SCI through positive AKT activity modulation. These results position MST2 as a potential therapeutic target for SCI, offering new insights into strategies for enhancing neuroregeneration and functional restoration.








Similar content being viewed by others
Data Availability
The datasets generated and analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
Mcdonald JW, Sadowsky C (2002) Spinal-cord injury. Lancet 359(9304):417–425. https://doi.org/10.1016/S0140-6736(02)07603-1
Mahar M, Cavalli V (2018) Intrinsic mechanisms of neuronal axon regeneration. Nat Rev Neurosci 19(6):323–337. https://doi.org/10.1038/s41583-018-0001-8
Huber AB, Schwab ME (2000) Nogo-A, a potent inhibitor of neurite outgrowth and regeneration. Biol Chem 381(5–6):407–419. https://doi.org/10.1515/BC.2000.053
Moore DL, Apara A, Goldberg JL (2011) Kruppel-like transcription factors in the nervous system: novel players in neurite outgrowth and axon regeneration. Mol Cell Neurosci 47(4):233–243. https://doi.org/10.1016/j.mcn.2011.05.005
Okada K, Tanaka H, Temporin K, Okamoto M, Kuroda Y, Moritomo H et al (2011) Akt/mammalian target of rapamycin signaling pathway regulates neurite outgrowth in cerebellar granule neurons stimulated by methylcobalamin. Neurosci Lett 495(3):201–204. https://doi.org/10.1016/j.neulet.2011.03.065
Wyatt LA, Filbin MT, Keirstead HS (2014) PTEN inhibition enhances neurite outgrowth in human embryonic stem cell-derived neuronal progenitor cells. J Comp Neurol 522(12):2741–2755. https://doi.org/10.1002/cne.23580
Shi Z, Zhou Z (2017) MST kinases in innate immune signaling. Cell Stress 2(1):4–13. https://doi.org/10.15698/cst2018.01.119
Rauch J, O’Neill E, Mack B, Matthias C, Munz M, Kolch W et al (2010) Heterogeneous nuclear ribonucleoprotein H blocks MST2-mediated apoptosis in cancer cells by regulating A-Raf transcription. Cancer Res 70(4):1679–1688. https://doi.org/10.1158/0008-5472.CAN-09-2740
Liu W, Wu J, Xiao L, Bai Y, Qu A, Zheng Z et al (2012) Regulation of neuronal cell death by c-Abl-Hippo/MST2 signaling pathway. PLoS ONE 7(5):e36562. https://doi.org/10.1371/journal.pone.0036562
Lessard-Beaudoin M, Laroche M, Loudghi A, Demers MJ, Denault JB, Grenier G et al (2016) Organ-specific alteration in caspase expression and STK3 proteolysis during the aging process. Neurobiol Aging 47:50–62. https://doi.org/10.1016/j.neurobiolaging.2016.07.003
Riechers SP, Butland S, Deng Y, Skotte N, Ehrnhoefer DE, Russ J et al (2016) Interactome network analysis identifies multiple caspase-6 interactors involved in the pathogenesis of HD. Hum Mol Genet 25(8):1600–1618. https://doi.org/10.1093/hmg/ddw036
Matsumoto H, Murakami Y, Kataoka K, Lin H, Connor KM, Miller JW et al (2014) Mammalian STE20-like kinase 2, not kinase 1, mediates photoreceptor cell death during retinal detachment. Cell Death Dis 5(5):e1269. https://doi.org/10.1038/cddis.2014.218
Chiba S, Ikeda M, Katsunuma K, Ohashi K, Mizuno K (2009) MST2- and Furry-mediated activation of NDR1 kinase is critical for precise alignment of mitotic chromosomes. Curr Biol 19(8):675–681. https://doi.org/10.1016/j.cub.2009.02.054
Kim M, Kim M, Lee MS, Kim CH, Lim DS (2014) The MST1/2-SAV1 complex of the Hippo pathway promotes ciliogenesis. Nat Commun 5:5370. https://doi.org/10.1038/ncomms6370
Song Z, Han X, Zou H, Zhang B, Ding Y, Xu X et al (2018) PTEN-GSK3beta-MOB1 axis controls neurite outgrowth in vitro and in vivo. Cell Mol Life Sci 75(23):4445–4464. https://doi.org/10.1007/s00018-018-2890-0
Xiao CL, Yin WC, Zhong YC, Luo JQ, Liu LL, Liu WY et al (2022) The role of PI3K/Akt signalling pathway in spinal cord injury. Biomed Pharmacother 156:113881. https://doi.org/10.1016/j.biopha.2022.113881
Read DE, Gorman AM (2009) Involvement of akt in neurite outgrowth. Cell Mol Life Sci 66(18):2975–2984. https://doi.org/10.1007/s00018-009-0057-8
Ding Y, Song Z, Liu J (2016) Aberrant LncRNA expression Profile in a contusion spinal cord Injury Mouse Model. Biomed Res Int 2016:9249401. https://doi.org/10.1155/2016/9249401
Kim B (2017) Western blot techniques. Methods Mol Biol 1606:133–139. https://doi.org/10.1007/978-1-4939-6990-6_9
Song Z, Han X, Zou H, Zhang B, Ding Y, Xu X et al (2018) PTEN–GSK3β–MOB1 axis controls neurite outgrowth in vitro and in vivo. Cell Mol Life Sci 75(23):4445–4464. https://doi.org/10.1007/s00018-018-2890-0
Basso DM, Fisher LC, Anderson AJ, Jakeman LB, Mctigue DM, Popovich PG (2006) Basso Mouse Scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J Neurotrauma 23(5):635–659. https://doi.org/10.1089/neu.2006.23.635
Alvarez Z, Kolberg-Edelbrock AN, Sasselli IR, Ortega JA, Qiu R, Syrgiannis Z et al (2021) Bioactive scaffolds with enhanced supramolecular motion promote recovery from spinal cord injury. Science 374(6569):848–856. https://doi.org/10.1126/science.abh3602
Vlahos CJ, Matter WF, Hui KY, Brown RF (1994) A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002). J Biol Chem 269(7):5241–5248
Gills JJ, Dennis PA (2009) Perifosine: update on a novel akt inhibitor. Curr Oncol Rep 11(2):102–110. https://doi.org/10.1007/s11912-009-0016-4
Romano D, Matallanas D, Weitsman G, Preisinger C, Ng T, Kolch W (2010) Proapoptotic kinase MST2 coordinates signaling crosstalk between RASSF1A, Raf-1, and Akt. Cancer Res 70(3):1195–1203. https://doi.org/10.1158/0008-5472.CAN-09-3147
Chen S, Fang Y, Xu S, Reis C, Zhang J (2018) Mammalian Sterile20-like kinases: Signalings and roles in Central Nervous System. Aging Dis 9(3):537–552. https://doi.org/10.14336/AD.2017.0702
Bellver-Landete V, Bretheau F, Mailhot B, Vallieres N, Lessard M, Janelle ME et al (2019) Microglia are an essential component of the neuroprotective scar that forms after spinal cord injury. Nat Commun 10(1):518. https://doi.org/10.1038/s41467-019-08446-0
Hu X, Huang J, Li Y, Dong L, Chen Y, Ouyang F et al (2022) TAZ induces Migration of Microglia and promotes neurological recovery after spinal cord Injury. Front Pharmacol 13:938416. https://doi.org/10.3389/fphar.2022.938416
Anderson MA, Burda JE, Ren Y, Ao Y, O’Shea TM, Kawaguchi R et al (2016) Astrocyte scar formation aids central nervous system axon regeneration. Nature 532(7598):195–200. https://doi.org/10.1038/nature17623
Xie C, Shen X, Xu X, Liu H, Li F, Lu S et al (2020) Astrocytic YAP promotes the formation of glia scars and neural regeneration after spinal cord Injury. J Neurosci 40(13):2644–2662. https://doi.org/10.1523/JNEUROSCI.2229-19.2020
Wang P, Geng J, Gao J, Zhao H, Li J, Shi Y et al (2019) Macrophage achieves self-protection against oxidative stress-induced ageing through the Mst-Nrf2 axis. Nat Commun 10(1):755. https://doi.org/10.1038/s41467-019-08680-6
Geng J, Sun X, Wang P, Zhang S, Wang X, Wu H et al (2015) Kinases Mst1 and Mst2 positively regulate phagocytic induction of reactive oxygen species and bactericidal activity. Nat Immunol 16(11):1142–1152. https://doi.org/10.1038/ni.3268
O’Neill E, Rushworth L, Baccarini M, Kolch W (2004) Role of the kinase MST2 in suppression of apoptosis by the proto-oncogene product Raf-1. Science 306(5705):2267–2270. https://doi.org/10.1126/science.1103233
Creasy CL, Chernoff J (1995) Cloning and characterization of a member of the MST subfamily of Ste20-like kinases. Gene 167(1–2):303–306. https://doi.org/10.1016/0378-1119(95)00653-2
Park SJ, Jin ML, An HK, Kim KS, Ko MJ, Kim CM et al (2015) Emodin induces neurite outgrowth through PI3K/Akt/GSK-3beta-mediated signaling pathways in Neuro2a cells. Neurosci Lett 588:101–107. https://doi.org/10.1016/j.neulet.2015.01.001
Zheng J, Shen WH, Lu TJ, Zhou Y, Chen Q, Wang Z et al (2008) Clathrin-dependent endocytosis is required for TrkB-dependent akt-mediated neuronal protection and dendritic growth. J Biol Chem 283(19):13280–13288. https://doi.org/10.1074/jbc.M709930200
Kim Y, Seger R, Suresh BC, Hwang SY, Yoo YS (2004) A positive role of the PI3-K/Akt signaling pathway in PC12 cell differentiation. Mol Cells 18(3):353–359
Kumar V, Zhang MX, Swank MW, Kunz J, Wu GY (2005) Regulation of dendritic morphogenesis by Ras-PI3K-Akt-mTOR and Ras-MAPK signaling pathways. J Neurosci 25(49):11288–11299. https://doi.org/10.1523/JNEUROSCI.2284-05.2005
Lim CS, Walikonis RS (2008) Hepatocyte growth factor and c-Met promote dendritic maturation during hippocampal neuron differentiation via the akt pathway. Cell Signal 20(5):825–835. https://doi.org/10.1016/j.cellsig.2007.12.013
Chen CH, Sung CS, Huang SY, Feng CW, Hung HC, Yang SN et al (2016) The role of the PI3K/Akt/mTOR pathway in glial scar formation following spinal cord injury. Exp Neurol 278:27–41. https://doi.org/10.1016/j.expneurol.2016.01.023
Cinar B, Fang PK, Lutchman M, Di Vizio D, Adam RM, Pavlova N et al (2007) The pro-apoptotic kinase Mst1 and its caspase cleavage products are direct inhibitors of Akt1. EMBO J 26(21):4523–4534. https://doi.org/10.1038/sj.emboj.7601872
Basu S, Totty NF, Irwin MS, Sudol M, Downward J (2003) Akt phosphorylates the yes-associated protein, YAP, to induce interaction with 14-3-3 and attenuation of p73-mediated apoptosis. Mol Cell 11(1):11–23. https://doi.org/10.1016/s1097-2765(02)00776-1
Wang K, Degerny C, Xu M, Yang XJ (2009) YAP, TAZ, and Yorkie: a conserved family of signal-responsive transcriptional coregulators in animal development and human disease. Biochem Cell Biol 87(1):77–91. https://doi.org/10.1139/O08-114
Ling P, Lu TJ, Yuan CJ, Lai MD (2008) Biosignaling of mammalian Ste20-related kinases. Cell Signal 20(7):1237–1247. https://doi.org/10.1016/j.cellsig.2007.12.019
Acknowledgements
The authors would like to express their gratitude to Nature Research Editing for the expert linguistic services provided.
Funding
This work was supported by grants from the National Science Foundation of China (Nos. 81972048, 81901247) and the Danyang Key Research and Development Program (Social Development) (Grant No. SSF202303).
Author information
Authors and Affiliations
Contributions
Conception and design: Hongming Zheng, Zhiwen Song and Jinbo Liu; Development of methodology: Hongming Zheng and Honghai Wang; Acquisition of data: Yi Xu, Xu Xu, and Zhenghuan Zhu; Analysis and interpretation of data: Hongming Zheng, Zhiwen Song and Jiawei Fang; Writing of the manuscript: Hongming Zheng and Zhiwen Song. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
The animal study was conducted in accordance with ethical standards and approved by the Ethic Committee of Animal Research in The Third Affiliated Hospital of Soochow University.
Consent to Participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Supplementary Material 1
: Figure S1. Validation of MST2 Knockdown Efficiency via Lentiviral shRNAs in Neuro 2A and HT22 cells. (a) Presentation of three shRNA constructs targeting MST2 (sh-MST2-1, sh-MST2-2, sh-MST2-3) used for lentiviral vector construction. (b) Diagram of the lentiviral vector backbones utilized. (c?f) Western blot showing shRNA knockdown efficiency in Neuro 2A and HT22 cells; GAPDH was used as an internal control. Efficacy is indicated by decreased MST2 protein levels, confirming successful gene silencing (**P < 0.01 vs. sh-control).
Supplementary Material 2
: Figure S2. Viability and NeuN Expression in Primary Neurons Following MST2 Knockdown (a) CCK-8 assay depicting the viability of primary neurons at 1, 3, 5, and 7 days post-infection with sh-MST2, showing no significant change compared to the sh-control group, indicating that MST2 knockdown does not adversely affect cellular viability. (b) Western blot analysis and quantification of NeuN expression in primary neurons at 7 days post-infection with sh-MST2. Actin is used as a loading control, demonstrating that NeuN levels remain consistent with the sh-control group, suggesting that neuronal identity and health are maintained after MST2 knockdown.
Supplementary Material 3
: Table S1. Primer sequence in this study.
Supplementary Material 4
: Table S2. Antibodies sheet.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zheng, H., Wang, H., Xu, Y. et al. MST2 Acts via AKT Activity to Promote Neurite Outgrowth and Functional Recovery after Spinal Cord Injury in Mice. Mol Neurobiol (2024). https://doi.org/10.1007/s12035-024-04158-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12035-024-04158-9