Abstract
Introduction
Comorbidities are significant patient factors that contribute to outcomes after surgery. There is highly variable collection of this information across the literature. To help guide the systematic collection of best practice data, the Australian Spine Registry conducted an evidence map to investigate (i) what comorbidities are collected by spine registries, (ii) how they are collected and (iii) the compliance and completeness in collecting comorbidity data.
Method
A literature search was performed to identify published studies of adult spine registry data reporting comorbidities. In addition, targeted questionnaires were sent to existing global spine registries to identify the maximum number of relevant results to build the evidence map.
Results
Thirty-six full-text studies met the inclusion criteria. There was substantial variation in the reporting of comorbidity data; 55% of studies reported comorbidity collection, but only 25% reported the data collection method and 20% reported use of a comorbidity index. The variation in the literature was confirmed with responses from 50% of the invited registries (7/14). Of seven, three use a recognised comorbidity index and the extent and methods of comorbidity collection varied by registry.
Conclusion
This evidence map identified variations in the methodology, data points and reporting of comorbidity collection in studies using spine registry data, with no consistent approach. A standardised set of comorbidities and data collection methods would encourage collaboration and data comparisons between patient cohorts and could facilitate improved patient outcomes following spine surgery by allowing data comparisons and predictive modelling of risk factors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many patients undergoing spine surgery have general health comorbidities. The most common age group for surgical interventions is people between 60 and 80 years of age [1], which is a rapidly increasing demographic in many First-World countries such as Australia. Within this cohort, there are a common range of comorbidities in patients undergoing spine procedures, which may contribute to outcomes following surgery. These comorbidities include cardiovascular disease, chronic pulmonary conditions, cerebrovascular disease, diabetes, renal disease, liver disease, dementia, cancer and depression [2, 3].
In an effort to monitor outcomes for spine surgery patients and drive quality improvement, clinical registries have been established in many countries that follow-up spine surgery patients. A purpose of clinical registries is usually to collect patient outcome data to help improve practice through a process of evaluating outcomes and providing feedback to participating surgeons and hospitals [4, 5]. Ideally, such data would be collected prior to surgery and then at regular follow-up time points, such as 12 months and 24 months post-surgery.
The Australian Spine Registry (ASR) was established in 2016 as a pilot project [6]. As at July 2022, the registry has 15 surgeons in 18 hospital sites across Australia and has recruited 3685 patients. This number is expected to increase as the registry matures and as more surgeons and their patients are recruited. The ASR collects demographic and diagnostic information and patient-reported outcomes (PROMS) including the Oswestry Disability Index (ODI), Neck disability Index (NDI) and the EuroQol Five Dimensions (EQ5D). These validated measures are well-accepted measures of functional disability and quality of life relevant to spine surgery [7,8,9,10]. As an evolving clinical registry, there is a valuable opportunity at this time to consider what comorbidity data are important to collect and what can help inform research and clinical outcomes.
A previous systematic review examined a number of spine registries globally and summarised their data collection [4]. This review highlighted the heterogeneity of the data collected and the high risk of bias in many published studies and potential selection bias due to the type of registry [4]. Spine registries may be national or institutional organisations. National registries may be voluntary or mandatory, secondary to government or insurer regulations surrounding quality control or audit. Multicentre institutional registries may carry a risk of selection bias as many are tertiary referral institutions with selected patients or are industry sponsored [4]. In addition, the previous review made several recommendations regarding optimal data collection in future spine registries. These recommendations included improvements to the organisation and methodological approach to spine registries, the use of patient-reported outcome measures (PROMS), improvements to analysis and reporting, and practical data management goals [11]. While a summary of the registries and data collected was presented in the previous review, information about the comorbidities collected by the respective registries was not detailed.
Comorbidities are significant patient factors that contribute to outcomes. Comorbidities in patients may be assessed using a comorbidity scale, such as the Charlson Comorbidity Index (CCI) or the Elixhauser Comorbidity Index [12]. Comorbidity scales can be used to estimate risk of mortality and post-operative complications in a systematic manner [13]. The majority of studies have shown that complications and hospital stay are associated with comorbidities. For example, higher CCI scores are associated with readmission after orthopaedic surgery [2]; a higher risk of mortality [14]; and predictive of post-operative outcome [15]. However, the relevance of individual comorbidities is unclear.
To help guide the systematic collection of best practice data in our registry and inform practice in other registries, this evidence mapping project aims to investigate (i) what comorbidities are collected in spine registries, (ii) how they are collected and (iii) the compliance and completeness of collected comorbidity data.
Methods
This evidence map reviewed the comorbidities collected by spine registries globally and identified where possible the measures and methods used for reporting comorbidities.
Inclusion criteria–data sources
In addition to published studies, we included grey literature, such as annual reports and conference proceedings.
The inclusion criteria for this evidence map were that the study (a) reported registry data, (b) reported comorbidity collection and data items, and (c) was limited to patients aged 18 and over.
We defined spine registry data as data collected in a multicentre activity which reports outcomes of spine surgery. This definition included national registries, registries collecting data from multiple hospital sites and surgical databases from multiple sources that include data for spine surgery outcomes. We defined compliance as the proportion of patients for whom data are entered. Completeness was defined as the extent of comorbidity data collected and reported.
Exclusion criteria
We did not include studies reporting data for people aged under 18 years due to a potential confounder with the consent process and disease spectrum. We also excluded studies from single disease-specific databases, data from single institutions, data related to spine trauma or emergency admission, non-English publications and those published before 2015. The rationale for limiting the search to results published since 2015 was that the previous systematic review by van Hoof and colleagues [4] had listed the registries and outcomes measured up to 2015. Since this time, new registries have been formed and we wanted to obtain a more contemporary view of the evidence about comorbidity measurement and inclusion within spine registries.
Healthcare settings
We included studies from all healthcare systems that contribute to spine registry data. Earlier work [4] has identified registries in numerous countries with differing healthcare systems. As such, we expected a range of healthcare settings and systems to be represented in the evidence map.
Outcomes–comorbidity measures
We included all studies that used the descriptor “comorbidity” or “comorbidities” with enough detail to determine what comorbidities had been collected and, if available, the method of collection. This pragmatic approach was taken as our knowledge of spine registry data suggested that there would considerable variation of collection and measurement methods.
Search strategy
The search strategy was designed to capture all publications that showed the comorbidities collected by spine registries and was based on the search used by van Hoof et al. [4], with additional terms to capture comorbidities. We included terms related to the words “spine”, “back pain”, “registry”, “comorbidity” and “surgery”. The terms were searched separately and then combined with the Boolean operator AND to include all terms.
The search was designed to retrieve a broad range of results and included all published research articles based on spine registry data and included conference abstracts. In addition, we used the search engines Google and Google Scholar to search for available registry annual reports and presentations from professional conferences that would not be available through the databases. Where annual reports were available, we included the most recently published version.
Ovid Embase (including Medline) was searched on 15 September 2021. The search included the fields of: title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword heading word, floating subheading word and candidate term word. The search was limited to English language results from 1 January 2015, to 15 September 2021. In addition, we used the same search strategy to search Scopus for any additional papers or conference abstracts. The search strategy is shown in a supplementary file (S1).
Additional primary data collection from established registries
Our knowledge of the literature based on spine registry data suggested that there may be limited information available in published articles, conference abstracts or published reports to detail the collection of comorbidity outcomes by spine registries. As such, we decided to write to established registries to directly solicit information. We established a list of spine registries, based on previously published work [4] and updated to reflect more recent additions. We searched for publicly available contacts for the relevant registries and augmented this with contact details for registry leaders known to the authors. A list of questions was drafted by one author and reviewed by two other authors. This list of questions is included as a supplementary file (S2). This data collection was approved by the Human Research Ethics Committee (HREC) of the participating university, HREC approval number 30985. The questions were then emailed to the relevant contact person of the respective registries.
Results
Articles, conference abstracts, annual reports and presentations
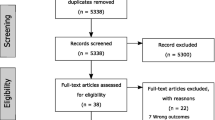
As shown in the PRISMA 2020 flow diagram (Fig. 1)[16], the search identified 172 records from Ovid Embase (including Medline), with a further 20 unique entries identified from Scopus. Following removal of duplicate records (n = 4), 188 records were screened on the abstract by one author (MQ). Ninety records were excluded based on the abstract. As such, 99 records were sought for retrieval. Twenty-two of these records were for conference abstracts, and as such, the full text was not available. Seventy-seven full-text articles were screened for inclusion by two authors (MQ and EA), with the data extraction spreadsheet reviewed by three authors (MQ, EA and MJ) until consensus was reached for inclusion. Following screening of the abstracts and full-text articles, 36 full-text articles and 4 conference abstracts were included in the evidence map. Reasons for exclusion included data from a single site (n = 22), a specialist database (n = 3), data from a registry other than spine (n = 7), insufficient comorbidity data (n = 4), paediatric data (n = 4) and systematic reviews (n = 1).
Five annual reports and 1 presentation were publicly available on the Internet. While 1 report (Australian Spine Registry) and 1 slide presentation (American Spine Registry) mentioned comorbidities, neither detailed the method of comorbidity collection.
Types of registries
Due to the inclusion criteria, studies represented different kinds of registries. There were 11 studies from national spine registries [17,18,19,20,21,22,23,24,25,26,27], 2 from combined registry data within a country [28, 29], 3 from an international spine registry [30,31,32], 4 from multicentre registries [11, 33,34,35], 15 from large surgical databases [36,37,38,39,40,41,42,43,44,45,46,47,48,49,50], 2 from a statewide registry [51, 52] and 3 where the type of registry was unclear [53,54,55]. Details of these registries are shown in Table 1.
Comorbidities collected and methods of collection
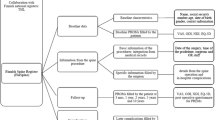
In the included published studies, the most commonly collected comorbidities were: neurological disease, cardiovascular disease, cancer, anxiety/depression, diabetes, smoking status, chronic obstructive pulmonary disease (COPD), cerebrovascular disease and obesity (Fig. 2). 55% of studies reported comorbidity collection. 20% of studies reported using a comorbidity index. Only 25% of these studies (10/40) explicitly stated how the actual data were collected and 38% of studies reported the comorbidity source, i.e. patient, EMR, surgeon, etc. (Fig. 2).
The method of comorbidity collection differed by registry. Of the 11 studies from national spine registries, SWEspine and DANEspine report use of patient-reported comorbidity data collected at baseline or following surgery, while others (e.g. NORspine) report use of surgeon reported data for individual patients [17, 21, 22]. One SWEspine study reported use of a comorbidity index calculated on healthcare utilisation in the 12 months prior to surgery [19]. The Canadian Spine Outcomes and Research Network (CSORN) registry employs local research coordinators who enrol the patients at each site and collect demographic and comorbidity data [20]. The 3 conference abstracts from established national spine registries (2 SWEspine, 1 CSORN) did not explicitly report the method of comorbidity data collection [25, 27, 56].
There were 3 studies from an international registry, Spine Tango [30,31,32]. Studies using data from this registry report the use of standardised forms to collect the American Society of Anaesthesiologists (ASA) grade but suggest that this registry does not collect other comorbidity data [31].
The 15 studies from large surgical outcomes database registries report employment of dedicated personnel and specific forms, and commonly utilise extraction from hospital records to populate the data at each site. Examples of this can be found in studies from the Quality Outcomes Database [38, 45], the Quality Outcomes Database Lumbar Registry [36, 39, 44, 47, 50] and the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) [37, 40,41,42,43, 46, 48, 49].
The one included statewide registry (Michigan Spine Surgery Improvement Collaborative (MSSIC)) reports collection of demographic and comorbidity data, but the collection method is not clear. One study from MSSIC reports comorbidity collection at baseline [51], while another reports collection via patient self-report 30 days after surgery [52].
The studies identified from multicentre registries reported comorbidity collection using the CCI or extraction to include the comorbidities covered by the CCI [11, 33,34,35]. One of these studies also documented the ASA grade [35].
The studies where the registry type was unclear did not state how the comorbidity data were collected [53,54,55], though one study reported that data were recorded for the diseases and/or states covered by the CCI [53].
An overview of the included studies, the comorbidities collected and the method of collection is shown in Table 2.
The extent of compliance and completeness in collecting comorbidities data
None of the included studies reported the compliance and success of comorbidity data collection from any type of spine registry.
Results–data collection from other spine registries
Of the 14 spine registries approached, we received responses from 7 (50%). These were the British Spinal Registry, the Norwegian registry for spine surgery (NORspine), Eurospine (Spine Tango), the Canadian Spine Outcomes and Research Network (CSORN), SweSpine, DaneSpine and the American Spine Registry (AmSR).
Registries reported similar variation to published studies regarding comorbidity collection. The most commonly collected comorbidities were: neurological disease, cardiovascular disease, cancer, anxiety/depression, diabetes, changes in daily activities, COPD and pain conditions (Fig. 2, Table 3). Recognised comorbidity indices were only used by 2/7 registries: the British Spine Registry (CCI) and CSORN (Quan Comorbidity Index). The American Spine Registry reports using an expanded dataset including the CCI where relevant to specific studies or reports. A further 3/7 of registries reported use of ASA grade entered by the operating surgeon in lieu of a recognised comorbidity index (SweSpine, DaneSpine, NORSpine). The use of patient-reported comorbidity data at baseline is common (SweSpine, DaneSpine, British Spine Registry CSORN). The American Spine Registry uses electronic medical record (EMR) extraction to report comorbidities identified by International Classification of Disease (ICD) codes, which are submitted to the registry by staff at participating institutions.
Similarly, as indicated in Table 3, the compliance and completeness for those entering the comorbidity information varied by registry. The British Spine Registry reported 100% compliance for patients entered into the registry, as the data fields are mandatory for both patients and clinicians. The Scandinavian registries report differing levels of compliance. For patient-reported comorbidities SweSpine reports 80% compliance at baseline, while DaneSpine reports “almost 100%”. NORspine reports difficulties in assessing compliance, as both patients and surgeons can choose a “no comorbidity” option. Difficulties in obtaining follow-up data were reported; Swespine reported 75% compliance at 1-year follow-up, while DaneSpine reported 80% compliance at 1 year and 45% compliance at 10 years. For ASA grade, there were also differences in compliance; NORspine reports 100% compliance, while DaneSpine reports approximately 50% compliance. The AMSR highlighted that it is difficult to ascertain whether “no reported comorbidities” represents patients with no comorbidities or the degree of missing data.
Discussion
Our findings have identified that there is variation in the methods of collection and the types of comorbidities collected in the published literature, with many studies not reporting these details. Our additional primary data collection from established registries was vital to our understanding regarding comorbidity collection in spine registries as many published studies provided scant detail about the methods of collection, the rationale for using a given method or the completeness and compliance associated with use.
Typically, the most complete comorbidity collection is done by large surgical databases, such as the American College of Surgeons NSQIP. These large multicentre databases or registries have dedicated staff who collect the comorbidity data directly from the patient at baseline or have access to patient medical records from either electronic hospital databases or national healthcare registers. In addition, for countries that have a nationalised healthcare system, or where medical records are available on a single national database, consistent comorbidity data are easier to collect. Canada, for example, has a universal, publicly funded, decentralised healthcare system where patient data are available through hospital EMRs. In Sweden, the use of personal identification numbers facilitates the linkage of patient data making the collection of specific comorbidities easier [57].
Communication with the established registries also identified that there was limited consistency in the comorbidities collected, confirming the findings from published studies. In spine surgery, the typical comorbidity measure is the CCI [12]. However, the CCI is now used to predict a variety of outcomes and to adjust for comorbidities. Furthermore, the CCI has been adapted and modified by researchers in relation to included conditions and the weighting of conditions [58] making comparisons across studies using different versions of CCI difficult. Some registries use ASA grades as a proxy for comorbidity measurement. The ASA classification was developed in 1942 and has undergone various revisions [59] with the latest amendment in 2020 [60]. It currently serves two functions: (a) to quantify the physiological reserve of a patient at the time they are about to undergo a surgical procedure and (b) as a healthcare billing tool [61]. Although not a formal comorbidity instrument or a formal predictor of perioperative risks, ASA is used to stratify the preoperative health status and for assessing risk of intra- and post-operative complications of spine surgery patients [62]. Although the ASA is a simple rating process, it is based on multiple factors and requires the expertise of a trained anaesthetist or surgeon. However, due to the subjective nature of scoring, studies have shown variability between surgeons’ and anaesthetists’ ASA scores [63, 64]. The inter-reliability of the ASA score, when different anaesthetists were compared, has been reported to be only “fair” [65].
However, a recent study evaluated the discriminatory ability of the ASA, the CCI and the modified Frailty Index (mFI) to predict adverse outcomes after lumbar fusion in 17,000 patients, finding that the most predictive comorbidity index was the ASA and the demographic factor of age [58]. This raises an interesting point about the need for collecting extensive comorbidity data.
The importance of age should not be overlooked. In 2019, the number of people globally over the age of 65 was 703 million with this number predicted to double by 2050 [66]. The proportion of older people undergoing spine surgery is also increasing with the age group where spine surgery is most frequently performed being 60–79 years [67]. The collection of comorbidity or “multimorbidity” data (multimorbidity defined as the presence of two or more chronic diseases [68]) is important for the assessment of risk and personalised management of patients undergoing spine surgery. To analyse spinal surgery outcomes, patient-reported outcomes, and to translate these outcomes into perioperative risk and healthcare costs, accurate collection of comorbidities is essential [68]. However, the methods used for collecting comorbidities in spine surgery literature is rarely reported, and the reporting of comorbidities is very heterogeneous [68, 69].
The use of comorbidity indices in large registries or national datasets, like NSQIP or CSORN, can also be influenced by the type of data the registry collects, the method of collection and the data completeness [58, 70]. Comorbidities which are collected directly from electronic hospital records, and coded by medical administrators, rely on the accuracy and definition of the comorbidities in the International Classification of Disease (ICD) handbook. As a consequence of updates to the ICD (the latest being ICD-10), registries and large dataset comorbidity definitions can change, and the indices they use need to be adapted for continued accuracy making longitudinal analyses difficult [70].
Strengths of this evidence map are that the literature was extensively searched and we subsequently collected primary data from international spinal registries to address the gaps in the published literature. Limitations of this evidence map include the low response rate from the primary data collection; of the 14 spine registries contacted, only 50% responded. However, the registries that responded represent a range of registries within different countries and healthcare systems, similar to the published literature. Another limitation is that our search and primary data collection were limited to the English language, so it is possible that there may be relevant studies and/or registries from non-English-speaking countries that were not included. Although potentially clinically important, the collection and relevance of risk adjustment factors such as patient education level or insurance status are not comorbidities and were therefore beyond the scope of this study.
Conclusion
This evidence map identified that publication of spine surgery comorbidity data either by registries or by smaller research groups is varied, with less than half of published studies reporting the methods of data collection and a low proportion reporting use of a comorbidity index. The quantity, type and collection method of comorbidity data, along with who should collect or report the data to accurately determine prediction of surgical risks and outcomes remains unclear. Without disclosure on the data source and method, the ability to directly compare information from different registries is extremely difficult. Due to the variability in published data, we recommend that these details be included in the literature to inform researchers of the types of methodologies used. For spine registries, it would be beneficial to have a standardised set of comorbidities and data collection methods to facilitate collaboration and data comparisons between patient cohorts. The use of a standardised set would help to build best practice in spine registries and may also facilitate improved patient outcomes following spine surgery by allowing predictive modelling of risk factors.
Data availability
The datasets generated during and/or analysed during the current study are not publicly available due to the inclusion of contact information of participants but are available from the corresponding author on reasonable request.
Change history
26 February 2023
Missing Open Access funding information has been added in the Funding Note.
References
Epstein NE (2011) Spine surgery in geriatric patients: sometimes unnecessary, too much, or too little. Surg Neurol Int 2:188
Voskuijl T, Hageman M, Ring D (2014) Higher Charlson comorbidity index scores are associated with readmission after orthopaedic surgery. Clin Orthop Relat Res® 472(5):1638–1644
Cram P, Landon BE, Matelski J, Ling V, Perruccio AV, Paterson JM et al (2019) Utilization and outcomes for spine surgery in the United States and Canada. Spine 44(19):1371
van Hooff ML, Jacobs WC, Willems PC, Wouters MW, de Kleuver M, Peul WC et al (2015) Evidence and practice in spine registries. Acta Orthop 86(5):534–544
Hoque DM, Sampurno F, Ruseckaite R, Lorgelly P, Evans SM (2017) Study protocol of an equivalence randomized controlled trial to evaluate the effectiveness of three different approaches to collecting patient reported outcome measures (PROMs) data using the prostate cancer outcomes registry-Victoria (PCOR-VIC). BMC Health Serv Res 17(1):75
Ahern S, Apos E, McNeil JJ, Cunningham J, Johnson M (2018) Monitoring outcomes in spine surgery: rationale behind the Australian spine registry. ANZ J Surg 88(10):950–951
McCormick JD, Werner BC, Shimer AL (2013) Patient-reported outcome measures in spine surgery. J Am Acad Orthop Surg 21(2):99–107
Vernon H (2008) The neck disability index: state-of-the-art, 1991–2008. J Manip Physiol Ther 31(7):491–502
EuroQol G (1990) EuroQol–a new facility for the measurement of health-related quality of life. Health Policy 16(3):199–208
Solberg TK, Olsen JA, Ingebrigtsen T, Hofoss D, Nygaard OP (2005) Health-related quality of life assessment by the EuroQol-5D can provide cost-utility data in the field of low-back surgery. Eur Spine J 14(10):1000–1007
Sciubba D, Jain A, Kebaish KM, Neuman BJ, Daniels AH, Passias PG et al (2021) Development of a preoperative adult spinal deformity comorbidity score that correlates with common quality and value metrics: length of stay, major complications, and patient-reported outcomes. Glob Spine J 11(2):146–153
Charlson M, Szatrowski TP, Peterson J, Gold J (1994) Validation of a combined comorbidity index. J Clin Epidemiol 47(11):1245–1251
Austin SR, Wong Y-N, Uzzo RG, Beck JR, Egleston BL (2015) Why summary comorbidity measures such as the Charlson comorbidity index and Elixhauser score work. Med Care 53(9):e65
Harris MB, Reichmann WM, Bono CM, Bouchard K, Corbett KL, Warholic N et al (2010) Mortality in elderly patients after cervical spine fractures. J Bone Jt Surg Am 92(3):567
Shinonara K, Ugawa R, Arataki S, Nakahara S, Takeuchi K (2021) Charlson comorbidity index is predictive of postoperative clinical outcome after single-level posterior lumbar interbody fusion surgery. J Orthop Surg Res 16(1):1–6
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg 88:105906
Andersen MØ, Fritzell P, Eiskjaer SP, Lagerbäck T, Hägg O, Nordvall D et al (2019) Surgical treatment of degenerative disk disease in three Scandinavian countries: an international register study based on three merged national spine registers. Glob Spine J 9(8):850–858
Holmberg ST, Salvesen ØO, Vangen-Lønne V, Hara S, Fredheim OM, Solberg TK et al (2020) Pain during sex before and after surgery for lumbar disc herniation: a multicenter observational study. Spine 45(24):1751–1757
Iderberg H, Willers C, Borgström F, Hedlund R, Hägg O, Möller H et al (2019) Predicting clinical outcome and length of sick leave after surgery for lumbar spinal stenosis in Sweden: a multi-register evaluation. Eur Spine J 28(6):1423–1432
Inculet C, Urquhart JC, Rasoulinejad P, Hall H, Fisher C, Attabib N et al (2021) Factors associated with using an interbody fusion device for low-grade lumbar degenerative versus isthmic spondylolisthesis: a retrospective cohort study. J Neurosurg Spine 35(3):299–307
Lagerbäck T, Fritzell P, Hägg O, Nordvall D, Lønne G, Solberg TK et al (2019) Effectiveness of surgery for sciatica with disc herniation is not substantially affected by differences in surgical incidences among three countries: results from the Danish, Swedish and Norwegian spine registries. Eur Spine J 28(11):2562–2571
Lønne G, Fritzell P, Hägg O, Nordvall D, Gerdhem P, Lagerbäck T et al (2019) Lumbar spinal stenosis: comparison of surgical practice variation and clinical outcome in three national spine registries. Spine J 19(1):41–49
Paulsen RT, Bouknaitir JB, Fruensgaard S, Carreon L, Andersen M (2018) Prognostic factors for satisfaction after decompression surgery for lumbar spinal stenosis. Neurosurgery 82(5):645–651
Sigmundsson FG, Jönsson B, Strömqvist B (2015) Outcome of decompression with and without fusion in spinal stenosis with degenerative spondylolisthesis in relation to preoperative pain pattern: a register study of 1624 patients. Spine J 15(4):638–646
Fritzell P, Hagg O, Strmqvist B, Knutsson B (2015) Patient reported value 1 year after surgery for lumbar disc herniation: predictors of outcome using the Swedish national spine register; SweSpine. Eurospine; Copenhagen, Denmark: European Spine Journal
Hebert J, Bigney E, Wedderkopp N, Richardson EA, Darling MA, Manson NA (2019) 179. Predictors of clinical outcome following surgery for lumbar spinal stenosis: a study of postoperative pain and disability trajectories. Spine J 19(9):S86–S87
Jonsson E, Hansson-Hedblom A, Fritzell P, Hägg O, Borgström F (2017) Surgical treatment outcome of lumbar disc herniation (LDH) in different ages. Value Health 20(9):A538
Eneqvist T, Nemes S, Brisby H, Fritzell P, Garellick G, Rolfson O (2017) Lumbar surgery prior to total hip arthroplasty is associated with worse patient-reported outcomes. Bone Jt J 99-B(6):759–765
Salmenkivi J, Sund R, Paavola M, Ruuth I, Malmivaara A (2017) Mortality caused by surgery for degenerative lumbar spine. Spine (Phila Pa 1976) 42(14):1080–1087
Sobottke R, Herren C, Siewe J, Mannion AF, Röder C, Aghayev E (2017) Predictors of improvement in quality of life and pain relief in lumbar spinal stenosis relative to patient age: a study based on the Spine Tango registry. Eur Spine J 26(2):462–472
Zehnder P, Aghayev E, Fekete TF, Haschtmann D, Pigott T, Mannion AF (2016) Influence of previous surgery on patient-rated outcome after surgery for degenerative disorders of the lumbar spine. Eur Spine J 25(8):2553–2562
Zehnder PAE, Fekete TF, Haschtmann D, Pigott T, Mannion AF 2016 The law of diminishing returns in surgery for degenerative spinal disorders: quantification of the effect of previous surgery on patient rated outcomes. Eurospine; Berlin, Germany: European Spine Journal
Protopsaltis TS, Diebo BG, Lafage R, Henry JK, Smith JS, Scheer JK et al (2018) Identifying thoracic compensation and predicting reciprocal thoracic kyphosis and proximal junctional kyphosis in adult spinal deformity surgery. Spine 43(21):1479–1486
Raad M, Jain A, Neuman BJ, Hassanzadeh H, Gupta MC, Burton DC et al (2018) Association of patient-reported narcotic use with short-and long-term outcomes after adult spinal deformity surgery: multicenter study of 425 patients with 2-year follow-up. Spine 43(19):1340–1346
Smith JS, Shaffrey CI, Klineberg E, Lafage V, Schwab F, Lafage R et al (2017) Complication rates associated with 3-column osteotomy in 82 adult spinal deformity patients: retrospective review of a prospectively collected multicenter consecutive series with 2-year follow-up. J Neurosurg Spine 27(4):444–457
Asher AL, Devin CJ, McCutcheon B, Chotai S, Archer KR, Nian H et al (2017) Patient characteristics of smokers undergoing lumbar spine surgery: an analysis from the quality outcomes database. J Neurosurg Spine 27(6):661–669
Boddapati V, Lee NJ, Mathew J, Held MB, Peterson JR, Vulapalli MM et al (2021) Respiratory compromise after anterior cervical spine surgery: incidence, subsequent complications, and independent predictors. Glob Spine J 12(8):1647–1654
Chan AK, Bisson EF, Bydon M, Glassman SD, Foley KT, Potts EA et al (2018) Women fare best following surgery for degenerative lumbar spondylolisthesis: a comparison of the most and least satisfied patients utilizing data from the quality outcomes database. Neurosurg Focus 44(1):E3
Cook CE, Garcia AN, Shaffrey C, Gottfried O (2020) The influence of unemployment and disability status on clinical outcomes in patients receiving surgery for low back-related disorders: an observational study. Spine Surg Relat Res 5(3):182–188
Cote DJ, Karhade AV, Larsen AM, Burke WT, Castlen JP, Smith TR (2016) United States neurosurgery annual case type and complication trends between 2006 and 2013: an American College of Surgeons National Surgical Quality Improvement Program analysis. J Clin Neurosci 31:106–111
Kalagara S, Eltorai AE, Durand WM, DePasse JM, Daniels AH (2018) Machine learning modeling for predicting hospital readmission following lumbar laminectomy. J Neurosurg Spine 30(3):344–352
Lee R, Lee D, Gowda NB, Iweala U, Weinreb JH, Falk DP et al (2020) Increased rates of septic shock, cardiac arrest, and mortality associated with chronic steroid use following anterior cervical discectomy and fusion for cervical stenosis. Int J Spine Surg 14(5):649–656
Malik AT, Jain N, Yu E, Kim J, Khan SN (2018) Is there a “sex effect” in 30-day outcomes after elective posterior lumbar fusions? World Neurosurg 120:e428–e433
McGirt MJ, Bydon M, Archer KR, Devin CJ, Chotai S, Parker SL et al (2017) An analysis from the quality outcomes database, part 1. Disability, quality of life, and pain outcomes following lumbar spine surgery: predicting likely individual patient outcomes for shared decision-making. J Neurosurg Spine 27(4):357–369
Mummaneni PV, Bisson EF, Kerezoudis P, Glassman S, Foley K, Slotkin JR et al (2017) Minimally invasive versus open fusion for grade I degenerative lumbar spondylolisthesis: analysis of the quality outcomes database. Neurosurg Focus 43(2):E11
Murphy ME, Kerezoudis P, Alvi MA, McCutcheon BA, Maloney PR, Rinaldo L et al (2017) Risk factors for dural tears: a study of elective spine surgery. Neurol Res 39(2):97–106
Park C, Cook CE, Garcia AN, Gottfried ON (2021) Discharge destination influences risks of readmission and complications after lumbar spine surgery in severely disabled patients. Clin Neurol Neurosurg 207:106801
Phan K, Kothari P, Lee NJ, Virk S, Kim JS, Cho SK (2017) Impact of obesity on outcomes in adults undergoing elective posterior cervical fusion. Spine 42(4):261–266
Sanford Z, Taylor H, Fiorentino A, Broda A, Zaidi A, Turcotte J et al (2019) Racial disparities in surgical outcomes after spine surgery: an ACS-NSQIP analysis. Glob Spine J 9(6):583–590
Laratta J, Carreon LY, Buchholz AL, Yew AY, Bisson EF, Mummaneni PV et al (2020) Effects of preoperative obesity and psychiatric comorbidities on minimum clinically important differences for lumbar fusion in grade 1 degenerative spondylolisthesis: analysis from the prospective quality outcomes database registry. J Neurosurg Spine 33(5):635–642
Macki M, Hamilton T, Lim S, Telemi E, Bazydlo M, Nerenz DR et al (2021) Disparities in outcomes after spine surgery: a Michigan spine surgery improvement collaborative study. J Neurosurg Spine 1(aop):1–9
Zakaria HM, Lipphardt M, Bazydlo M, Xiao S, Schultz L, Chedid M et al (2020) The preoperative risks and two-year sequelae of postoperative urinary retention: analysis of the Michigan spine surgery improvement collaborative (MSSIC). World Neurosurg 133:e619–e626
Kushioka J, Takenaka S, Makino T, Sakai Y, Kashii M, Iwasaki M et al (2020) Risk factors for in-hospital mortality after spine surgery: a matched case-control study using a multicenter database. Spine J 20(3):321–328
Kobayashi K, Imagama S, Ando K, Ishiguro N, Yamashita M, Eguchi Y et al (2017) Complications associated with spine surgery in patients aged 80 years or older: Japan Association of Spine Surgeons with Ambition (JASA) multicenter study. Glob Spine J 7(7):636–641
Kay HF, Chotai S, Wick JB, Stonko DP, McGirt MJ, Devin CJ (2016) Preoperative and surgical factors associated with postoperative intensive care unit admission following operative treatment for degenerative lumbar spine disease. Eur Spine J 25(3):843–849
Hebert JBE, Wedderkopp N, Richardson EA, Darling MA, Manson NA 2019 Predictors of clinical outcome following surgery for lumbar spinal stenosis: a study of postoperative pain and disability trajectories. In: 34th annual meeting of the North American Spine Society. Spine Journal, Chicago
Allin SMG, Peckham A (2020) University of Toronto. International health care system profiles. University of Toronto, Policies NAOoHSa, Canada
Ondeck NT, Bohl DD, Bovonratwet P, McLynn RP, Cui JJ, Shultz BN et al (2018) Discriminative ability of commonly used indices to predict adverse outcomes after poster lumbar fusion: a comparison of demographics, ASA, the modified Charlson Comorbidity Index, and the modified frailty index. Spine J 18(1):44–52
Knuf KA-O, Maani CV, Cummings AK. Clinical agreement in the American Society of Anesthesiologists physical status classification. (2047-0525 (Print))
ASA Physical Status Classification System. 2020. Accessed 13 Dec 2020
Schupper AJ, Shuman WH, Baron RB, Neifert SN, Chapman EK, Gilligan J et al (2021) Utilization of the American Society of Anesthesiologists (ASA) classification system in evaluating outcomes and costs following deformity spine procedures. Spine Deform 9(1):185–190
Mannion AF, Bianchi G, Mariaux F, Fekete TF, Reitmeir R, Moser B et al (2020) Can the Charlson comorbidity index be used to predict the ASA grade in patients undergoing spine surgery? Eur Spine J 29(12):2941–2952
Li G, Walco JP, Mueller DA, Wanderer JP, Freundlich RE (2021) Reliability of the ASA physical status classification system in predicting surgical morbidity: a retrospective analysis. J Med Syst 45(9):83
Kwa CXW, Cui J, Lim DYZ, Sim YE, Ke Y, Abdullah HR (2022) Discordant American Society of Anesthesiologists physical status classification between anesthesiologists and surgeons and its correlation with adverse patient outcomes. Sci Rep 12(1):7110
Sankar A, Johnson SR, Beattie WS, Tait G, Wijeysundera DN (2014) Reliability of the American Society of Anesthesiologists physical status scale in clinical practice. Br J Anaesth 113(3):424–432
Nations U (2019) World population ageing 2019: highlights. In: Department of Economic and Social Affairs PD
Mannion AF, Fekete TF, Porchet F, Haschtmann D, Jeszenszky D, Kleinstuck FS (2014) The influence of comorbidity on the risks and benefits of spine surgery for degenerative lumbar disorders. Eur Spine J 23(Suppl 1):S66-71
Cavalli L, Angehrn L, Schindler C, Orsini N, Grob C, Kaufmann M et al (2022) Number of comorbidities and their impact on perioperative outcome and costs—a single centre cohort study. Swiss Med Wkly 152:w30135
Bays A, Stieger A, Held U, Hofer LJ, Rasmussen-Barr E, Brunner F et al (2021) The influence of comorbidities on the treatment outcome in symptomatic lumbar spinal stenosis: a systematic review and meta-analysis. N Am Spine Soc J 6:100072
Ludvigsson JF, Appelros P, Askling J, Byberg L, Carrero JJ, Ekstrom AM et al (2021) Adaptation of the Charlson comorbidity index for register-based research in Sweden. Clin Epidemiol 13:21–41
Acknowledgements
The authors would like to acknowledge the following people and institutions for their valuable time and input in answering this study’s questionnaire: Diane Ziegenhorn, PT, DPT, MHA, Manager, Registry Programs & American Spine Registry Director. Greg McIntosh, MSc Canadian Spine Outcomes and Research Network, Director of Research Operations. Karen Højmark Hansen, Research Coordinator Spine surgery Research Unit, Spine Center of Southern Denmark. Emin Aghayev, MD MSc Project manager SIRIS Spine, Senior Advisor Spine Tango, EUROSPINE, The Spine Society of Europe. Peter Fritzell, Futurum Academy for Health and Care, Hus B4, Lanssjukhuset Ryhov, 553 05 Jonkoping, Sweden. Professor Tor Ingebrigtsen, University Hospital of North Norway, Department of Neurosurgery, ENT and Ophtalmology. Corri Conrad, Amplitude Clinical Registry for the British Spine Registry. The authors would also like to acknowledge Margaret Johnson for editorial review of the manuscript.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. No specific funding was received for this work. The work was partially supported by the HCF Research Foundation Grant 2019 to the Spine Society of Australia. MQ is supported by an Australian Government Research Training Program (RTP) scholarship. The funder(s) played no part in the study design, collection or interpretation of data, manuscript preparation or decision to publish.
Author information
Authors and Affiliations
Contributions
MJ and EA conceived the study. MQ, EA and MJ contributed to the design of the study. MQ provided methodological input, and MJ provided clinical input during the protocol phase. MQ designed the search strategy for the literature search and EA and MJ developed the questionnaire. MQ, EA and MJ appraised the quality of the literature and performed the data extraction. They also analysed the data both for the evidence map and for the questionnaire responses. MQ and EA drafted the manuscript. MQ drafted the supplementary material. All the authors critically revised the manuscript, read and approved the final version.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare with respect to this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Quigley, M., Apos, E., Truong, TA. et al. Comorbidity data collection across different spine registries: an evidence map. Eur Spine J 32, 753–777 (2023). https://doi.org/10.1007/s00586-023-07529-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-023-07529-3