Abstract
In the last 3 years of the pandemic situation, SARS-CoV-2 caused a significant number of deaths. Infection rates for symptomatic and asymptomatic patients are higher than that for death. Eventually, researchers explored that the major deaths are attributed to several comorbidity factors. The confounding factors and gender-associated infection/death rate are observed globally. This suggests that SARS-CoV-2 selects the human system recognizing the internal comorbid environment. This article explored the influences of hypertension, diabetes, cardiovascular, and renovascular disorders in COVID-19 severity and mortality. Brief mechanistic layouts have been presented here, indicating some of the comorbidity as the critical determinant in the COVID-19 pathogenesis and related mortality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coronavirus disease (COVID-19) is a worldwide pandemic that is brought on by the SARS-CoV-2 coronavirus, which first appeared in Wuhan, China, then spread incredibly swiftly to more than 180 other nations. SARS-CoV-2 is rapidly spreading all over the world, it is projected that patient characteristics, including age, sex, and comorbidity, might be more vulnerable to either an increased risk of infection or an increased death. According to certain reports, COVID-19 was higher in males than females (Huang et al. 2019; Chen et al. 2019), while patients who were somewhat older than 50 years had a higher rate of COVID-19 confirmed cases, according to some studies (Yang et al. 2020; Zhang et al. 2020a). Comorbidities are defined in medicine as the simultaneous presence of multiple underlying illnesses in one individual. Each condition is considered comorbidity, and sometimes comorbidities could be present in the form of mental or physical conditions. The best examples of comorbidities are when a person is suffering from diabetes and asthma, or when the person is suffering from hypertension and clinical depression. Comorbidities are generally non-communicable and contribute to nearly two-thirds of the annual deaths around the world. The prevalence of comorbidities in patients with COVID-19 was also highly variable in many reports (Li et al. 2020). A recent study showed that among COVID-19 patients, fever (88.8%) was the most prevalent symptom, followed by fatigue (33%) and dry cough (68%) (Paudel 2020). Another symptom included productive shortness of breath, or SOB (17%), cough (28.5%), sore throat (11.4%), muscle pain (14%), and headache (10%) (Paudel 2020). The least common symptoms were nausea and vomiting (4.1%), diarrhea (4.4%), abdominal pain (0.16%), rhinorrhea (3.2%), and chest pain (0.11%) (Paudel 2020). The progression of the disease COVID-19 is linked to a number of comorbidities. Patients with COVID-19 who have diabetes, hypertension, HIV, chronic obstructive pulmonary disease (COPD), cardiovascular diseases (CVD), malignancies, and other comorbidities may develop a condition that is life-threatening. Comorbidity is linked to severe medical conditions, higher medical expenses, and more difficult clinical management. SARS-CoV-2 enters into cells using ACE2 receptors that are present on the surface of the host cell. Certain comorbidities are associated with a strong ACE2 receptor expression and higher release of proprotein convertase that enhances the viral entry into the host cells. The comorbidities lead the COVID-19 patient into a violent infectious circle of life and are substantially associated with significant morbidity and mortality. COVID-19 infection is more likely in people with underlying serious medical disorders like high blood pressure, hyperglycemia, lung, kidney, and liver illness, smoking, cancer patients receiving chemotherapy, transplant recipients, and individuals taking steroids persistently (CDC. Coronavirus (COVID-19): symptoms of Coronavirus 2020). Cardiovascular and cerebrovascular conditions (11%), hypertension (15%), and diabetes (9%), among other conditions, were the most prevalent comorbidities found in these patients (Paudel 2020; Zhou et al. 2020). The less common comorbidities were coexisting infection with HIV and hepatitis B (1.5%), malignancy (1.5%), respiratory illnesses (1.4%), renal disorders (0.8%), and immunodeficiencies (0.01%) (Paudel 2020). Comorbidities may be a risk factor for COVID-19 infection given the high percentage of COVID-19 patients and other disorders among admitted ICU cases (Wang et al. 2020). Comorbidity may also relate to reduced immune function. For instance, in diabetic patients, natural immune function may be significantly diminished, which may restrict the body to produce respective antibodies against any infection (Berbudi et al. 2020). Also, polypharmacy and comorbidity are interrelated and dependable on each other. In order to better direct clinical therapy, there has not yet been a systematic review that thoroughly examines whether the presence of common comorbidities increases COVID-19 patients’ risk, to guide clinical practice better. Therefore, this research was designed to review the association of different comorbidities with the risk of mortality by estimating aggregated risk in patients with COVID-19.
ACE2
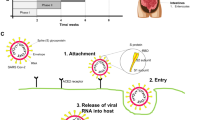
A lot of researchers are interested in a particular protein that allows SARS CoV-2 to infect human cells. This specific protein is called “angiotensin-converting enzyme 2 or ACE2 receptor”. ACE2 receptor serves cellular entry point for COVID-19 to hook into and infect a wide range of human cells. ACE2 is a protein widely expressed at the surface of many cells and also acts as a cellular doorway for the SARS-CoV2 virus, which causes COVID-19. The 805 amino acids in ACE2 are organized into a single extracellular catalytic domain. ACE2 is a type I zinc trans-membrane metallocarboxypeptidase with variable homology to ACE and an enzyme that cut larger protein angiotensinogen into small proteins that then go on to regulate functions in the other cells. ACE2 can offset the renin-angiotensin system’s (RAS) detrimental effects in a variety of illnesses (Donoghue et al. 2000). ACE2 is present in many cell types and tissue, such as the blood vessels, lungs, kidneys, heart, gastrointestinal tract, testes, and liver. It is present in all tissues’ endothelial cells from small and large arteries and veins, as well as in epithelium in the lungs, nose, and mouth, which create protective barriers. In the lungs, ACE2 is highly rich with type 2 pneumocytes, an important cell type present in chambers within the alveoli, where oxygen is absorbed and waste carbon dioxide is released. ACE2 serves as a co-receptor for nutrient absorption, particularly for amino acid resorption from meals, on the surface of intestinal epithelial cells in the kidney (Hashimoto et al. 2012). In the nasopharynx and nasal and oral mucosa, we discovered ACE2 expression in the basal layer of the non‐keratinizing squamous epithelium. The basal cell layer of the epidermis and the basal cell layer of the hair follicles in the skin both contained ACE2. Although ACE2 has similarities with ACE, its function is different from ACE; ACE2 releases a single amino acid (monocarboxypeptidase), whereas ACE cleaves a C-terminal dipeptide from its substrate (dipeptidylpeptidase) (Donoghue et al. 2000; Tipnis et al. 2000). Angiotensin I (Ang I) is converted by ACE into strong vasoconstrictor Ang II and ACE2 then uses its carboxypeptidase activity to cleave Ang II and hydrolyzes it into heptapeptide called Ang (1–7) (Turner et al. 2004). ACE2 also cleaves Ang I to the inactive peptide Ang (1–9), which is then converted into vasodilator peptide Ang (1–7) by ACE, competing with Ang I and decreasing Ang II (Arendse et al. 2019). Additionally, ACE2 directly breaks down Ang II to generate Ang 1–7 with much higher efficiency than converting Ang I to Ang 1–9. Ang 1–7 then binds to the MasR receptor, which was initially thought to be an orphan receptor due to the suppression of Ang 1–7 effects caused by the application of a MasR antagonist (Alenina et al. 2008). ACE2 also hydrolyzes a number of other peptides, including apelin‐13, bradykinin, neurotensin‐ (1–11), β‐casomorphin‐(1–7), dynorphin A‐ (1–13), and ghrelin (Vickers et al. 2002). In addition to serving as an amino transporter, ACE2 acts as a functional receptor for severe acute respiratory syndrome coronaviruses (SARS-CoV). The physiological purpose of ACE2 is to act as a counterbalance to ACE. ACE cleaves the angiotensin I hormone into the vasoconstricting angiotensin II. ACE2, in turn, cleaves the carboxyl-terminal amino acid phenylalanine from angiotensin II and converts it into the vasodilator angiotensin (1–7). Other peptides that ACE2 can break down include neurotensin, [des-Arg9]-bradykinin, apelin, dynorphin A, and ghrelin (Turner 2015). Multiple mechanisms control ACE2, including transcriptional, posttranscriptional (miRNA and epigenetic), and posttranslational through its shedding from the cell surface. The stronger binding of SARS CoV-2 with ACE2 than SARS-CoV may help to explain why SARS CoV2 infection has a more widespread effect than SARS CoV (Shang et al. 2020; Yan et al. 2020). A higher level of soluble ACE2 expression in the blood may result from increased ACE2 expression on the cell surface, which may actually bind to most SARS-CoV-2 and prevent it from interacting with the membrane-bound receptor (Fig. 1). Tissue ACE2 downregulation and RAS imbalance might contribute to the advancement of multi-organ damage attributed to SARS-CoV-2 infections (Wang et al. 2020). A decreased level of ACE2 secondary to SARS-CoV-2 infection followed by an increase in Ang II can exacerbate cardiovascular disease symptoms or promote further disease complications (Oudit et al. 2009; Aksoy et al. 2020).
Obesity, hypertension, and diabetes are among the most prevalent comorbidities that are frequently linked to catastrophic outcomes brought on by due to SARS-CoV-2 infections (Obukhov et al. 2020; Richardson et al. 2020). The level of ACE2 expression in the liver and kidney has been shown to increase when patients take ARB and ACEi medications, but these patients do not appear to have an increased risk of developing SARS-CoV-2 infection or suffering from severe disease. Instead, it appears that reduced ACE2 activity may be a key role in increased disease severity rather than the initial high levels of ACE2 expression leading to worsened prognosis.
ACE2 in renin angiotensin system
The complex hormonal axis known as RAAS, which plays a role in blood pressure control, sodium reabsorption, fibrosis, and inflammation, is made up of renin, aldosterone, and angiotensin (Ghazi and Drawz 2017). RAAS imbalance or modification can cause numerous disorders, including heart failure, hypotension, atherosclerosis, and diabetes, respectively (Tikellis and Thomas 2012). The renin-angiotensin system (RAS) is a peptidergic system that maintains the homeostatic control of the cardiovascular and renal systems and controls extracellular fluid volume. Inhibition of the RAS plays a central role in alleviating the increased mortality and morbidity of patients with heart failure (HF) (Givertz 2001; Zaman et al. 2002). Angiotensin II (Ang) is produced by a series of enzyme activities within the RAS. Renin, an aspartyl proteinase secreted by the kidney into the bloodstream, produces Ang I in the first phase by cleaving hepatic peptide angiotensinogen in the blood. Ang I is then hydrolyzed by angiotensin-converting enzyme (ACE) in the second stage, resulting in the octapeptide Ang II (Fig. 1). This biologically active peptide acts on both Ang II type 1 and type 2 receptors (AT1R and AT2R) (Bader and Ganten 2008 Jun). Ang II promotes inflammation, vasoconstriction, oxidative stress, and salt and water reabsorption via the activation of AT1R. RAS blockers increase angiotensin II, which is a substrate for ACE2. By regulating vasoconstriction, ACE2 is a component of RAS, which maintains blood pressure, fluid and electrolyte balance, and reabsorption of sodium in the kidney (Song et al. 2019). The interaction between ACE2 and angiotensin II could induce a conformational change in the RBD domain of ACE2 (Towler et al. 2004). The human body contains two functional forms of ACE2: the tissue-bound or membranous form and the soluble or circulating form (Vaduganathan et al. 2020; Batlle et al. 2020). The extracellular domain of membranous ACE2 has a receptor for attachment with spike (S) protein of SARS-CoV-2, allowing for viral entry (Fig. 1). Since the soluble form of ACE2 lacks the transmembrane anchor and it can freely circulate in the blood. According to Monteil et al. (Monteil et al. 2020), the SARS-CoV-2-ACE2 complex first binds to the host cells’ cellular membrane through the spike protein of the virus before entering the cells themselves. The virus would release into host cells upon membrane fusion. The ACE2 on the cellular membrane of the host cells showed a decline, and RAS showed an imbalance, which triggered the inflammatory reactions. Following the initial entry of SARS-CoV-2 into the cells through ACE2, the virus further controls ACE2 expression, reducing the protective properties of the enzyme Ang II is transformed into Ang (1–7) and Ang-I into inactive Ang by ACE2 (1–9). The unopposed action of Ang-II through its AT1 receptor is caused downregulation of ACE2. As a result, local RAS activation is caused by increased Ang II activity, which in turn causes organ damage seen with COVID-19 (Vaduganathan et al. 2020). By preventing its manufacture and receptor, respectively, ACEi and ARB work to counteract the Ang effects. Additionally, ACE-I/ARB may increase the amount of the soluble form of ACE2 and prevent the virus from entering the host cell. ARBs and ACE-Is both have differing effects on Ang-II, with the latter blocking its receptor while the former prevents its production (reduction in Ang-II levels) (no effect on levels of Ang-II). ACE-I/ARB will protect against this organ damage by (1) possible upregulation of ACE2 activity, (2) reduced levels of Ang-II (by ACE-Is), and (3) inhibiting the ATI receptor of Ang-II. ACE-I/ARB will guard against this organ harm (by ARBs). Increased levels of ACE2, with the theoretical chance that elevated the levels of ACE2 generated by ACEi/ARB, could facilitate SARS-CoV-2 invasion and amplify the amount of organ damage brought on by this unique coronavirus.
Effect of ACE2 on salt balance
It is believed that angiotensin-converting enzyme 2 (ACE2) balances off ACE by the breakdown of Ang II and producing Ang (1–7). Although the kidney contains substantial levels of these enzymes, accounts of how they are controlled vary. In order to better understand how renin–angiotensin–aldosterone-aldosterone system (RAAS) pharmacological changes (ACE inhibition) in activity and physiological (low-sodium diet) changes (ACE inhibition) affect renal ACE and ACE2 expression, renal ACE and ACE2 expression were both examined. Those high in salt, glucose, and fat have the potential to quickly modify the activity and expression of ACE2. In mice, a high salt diet raised ACE2 activity in the urine, but a low salt diet lowered it (Wysocki et al. 2013). In fact, a high-salt diet was observed to raise the ratio of ACE/ACE2 in the glomeruli, which led to renal dysfunction by causing oxidative stress (Bernardi et al. 2012). Additionally, ACE2 was linked to the pathophysiology of hypertension, which was closely related to a high-salt diet. Consuming excessive amounts of salt made hypertension more common in people with the ACE2 rs2285666 TT and rs714205 GG genes (He et al. 2017). According to studies (Crackower et al. 2002; Raizada and Ferreira 2007; Tikellis et al. 2011), the ratio ACE/ACE2 determines the tissue levels of Ang II, and deficiency in ACE2 leads to an increase in Ang II levels (Crackower et al. 2002).
ACE2 appears to counteract the effects of Ang II and produce beneficial Ang 1–7. In both experimental and human renal illnesses, Ang II levels are elevated in injured tubules, making it a potential mediator of renal damage. There is strong evidence that renin is the rate-limiting step for the activation of Ang II via its control of Ang I formation (Hollenberg 2010). For instance, variations in salt intake alter Ang II activity largely via changes in renin release and plasma renin activity. Little is known regarding ACE2 activity changes brought on by physiological disturbances such as dietary salt intake. Since Ang II is controlled by the enzyme ACE2, changes in ACE2 would be anticipated anytime ACE or Ang II levels fluctuate. To maintain the Ang II level at a steady state, the production driven by ACE and the degradation caused by ACE2 need to be coordinated in many comorbid conditions (Fig. 1). When Ang II formation increases as a result of Ang I formation, an increase in ACE would also be expected. Because Ang II would otherwise increase without a checkpoint in this situation, ACE2 would eventually rise to encourage its degradation. It is likely that the rise in ACE2 levels is delayed or does not coincide with the rise in ACE activity, maintaining transiently high Ang II levels similar to those seen in participants after a low-salt diet. On the other hand, one would anticipate a decrease in ACE, followed by a fall in ACE2, if Ang II production reduces, as in a high-salt diet.
Effect of ACE2 on hypertension
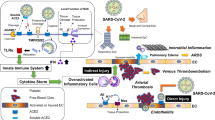
The protein ACE2 is widely known for its role in hypertension. In three strains of genetically hypertensive rats, the ACE2 gene maps to a quantitative trait locus on the X chromosome, suggesting that ACE2 may be the gene that causes hypertension. It is theorized that an imbalance between the tissue’s ACE and ACE2 proteins causes enhanced ACE2 expression to guard against increased blood pressure and ACE2 deficiency to exacerbate hypertension. Through negatively regulating the renin-angiotensin system, which includes the pressor-hypertrophic route of ANG II and the depressor-antiproliferative effects of Ang-(1–7) (Li et al. 2019; Ferrario et al. 2005), ACE2 adjusts blood pressure and maintains blood pressure homeostasis. Angiotensin-converting enzyme inhibitors (ACEi) or angiotensin receptor blockers (ARBs) are two commonly administered common antihypertensive medicines that target RAS. Hypertension may be the primary co-morbidity and a potential risk factor for more severe clinical outcomes of COVID-19 (Grasselli et al. 2020). Additionally, ACEi and ARBs are used to treat hypertension which upregulates the amount of ACE2 level (Li et al. 2017). It was once believed that aberrant blood pressure management would come from the disruption of the delicate balance between ACE and ACE2 (Brunner 2001). The effects of the renin–angiotensin system on cardiac remodeling, vasoconstriction, vasopressin synthesis and release, and sympathetic outflow plays a significant role in the regulation of blood pressure and volume homeostasis (Allen et al. 2000). The primary physiologically active effector peptide of the RAS, Ang II, works primarily by interacting with the angiotensin II type-1 receptor (AT1R), helping to control blood pressure (Ferrario 1998). Ang II is transformed by ACE2 into Ang (1–7), which works at the Mas receptor to lower blood pressure and lessen fibrosis and inflammation (South et al. 2019). Although the peripheral infusion of circulating Ang II can boost neuronal activity and it is also associated with autonomic and cardiovascular control, which can result in sympathetic hyperactivity and neurogenic hypertension (Ferguson and Bains 1997; Davern and Head 2007; McKinley et al. 1998). The main effects of Ang-(1–7) are to improve sodium and water excretion, increase nitric oxide generation and vasodilation, and decrease sympathetic nervous system tone (Sampaio et al. 2007). The main consequences of the ACE/Ang II/Ang II type 1 receptor pathway are countered by this, including vasoconstriction, sodium and water reabsorption, increased sympathetic nervous system tone, and increased oxidative stress, which results in inflammation and fibrosis (Masi et al. 2019). Most tissues, including the heart, lungs, vasculature, kidney, and intestines, coexpress both routes. Thus, the balance between the two main RAAS pathways, ACE2/Ang-(1–7) and ACE/Ang II, plays a crucial role in cardiovascular and renal illness as well as the onset, progression, and remission of hypertension in both children and adults (Fig. 2). ACE inhibitors and angiotensin receptor blockers may upregulate ACE2 expression, so increasing the availability of target molecules for SARS-CoV-2, according to some animal studies. Although it might not be enough to offset the harmful effects of Ang II, ACE2 may act as a compensatory mechanism to maintain Ang (1–7) levels in response to hypertension-induced cardiac hypertrophy in this strain. Uncontrolled blood pressure and a high case fatality rate are also linked to COVID-19 infection (CFR). Young animals, including humans, exhibit low ACE2 levels [especially before puberty], and male animals have shown higher ACE2 levels, and high ACE2 levels are associated with both hypertension and diabetes (Thatcher et al. 2012). Hypertensive patients with SARS-CoV-2 infection were shown to have 2.27- and 3.48-fold higher risks of severity and fatality compared to the COVID-19 cases without hypertension, respectively. ACE2 has been measured as a protective factor against increases in blood pressure (Fig. 2). Therefore, it is hypothesized that the binding of SARS-COV-2 to ACE2 can decrease the physiological function of ACE2, which in turn could cause immediate unfavorable effects of hypertension such multi-organ dysfunction (Tipnis et al. 2000). Age was a significant risk factor for the severity and fatality of COVID-19, according to epidemiological data of SARS-CoV-2 infection, which revealed that severe COVID-19 cases were more likely to be older patients with underlying comorbidities (such as diabetes mellitus and hypertension) (Zhou et al. 2020; Guan et al. 2020). According to recent data from the China CDC, individuals over the age of 80 had the highest case fatality rate of any age group at 14.8% (Novel Coronavirus Pneumonia Emergency Response Epidemiology Team 2020). In both the age 50 years and 50 years groups, hypertension was strongly linked to the severity and fatality of SARS CoV-2 infection, indicating that it can independently raise the risk of disease severity and predict unfavorable outcomes of SARS CoV-2 infection (Fig. 2). Based on the hypothesis that ACE2 can be increased by ACE inhibitors or ARBs, ACE inhibitors or ARBs may have negative impacts on COVID-19 patient morbidity and mortality. Patients with high blood pressure typically receive treatment with ACE2 inhibitors and ARBs. These inhibitors boost the expression of the ACE2 receptor when administered in high doses, which increases SARS-CoV-2 infection (Fang et al. 2020).
Effect of ACE2 on diabetes
A metabolic condition known as diabetes mellitus is characterized by hyperglycemia and insufficient endogenous insulin production. It has become a major health concern and is predicted to be the fifth most common cause of death worldwide (Roglic et al. 2005). Renal disease, myocardial infarction, hypertension, and stroke are all more likely to develop in people with diabetes (Grundy et al. 1999). For the treatment and prevention of diabetic nephropathy, a progressive condition characterized by proteinuria, thickening of the glomerular basement membrane, mesangial matrix expansion, and associated with chronic renal failure, ACE2 may be a significant target. In kidneys, ACE2 is largely localized in tubular and glomerular epithelial cells (Fig. 2). In both mice and people with type 2 diabetes, there may be a correlation between decreased ACE2 expression in glomerular epithelial cells and increased ACE expression in diabetic kidney disease. Additionally, it has been demonstrated that ACE2 genetic ablation and pharmacological ACE2 inhibition both induce albuminuria, mesangial matrix deposition, glomerular damage, and fibronectin production. Increased albuminuria and glomerular damage are brought on by ACE2 downregulation, which may also result in excessive Ang II buildup, particularly at the glomerular level (Ye et al. 2006). Increased protein excretion is caused by Ang II’s interference with a glomerular barrier function. Ang II inhibitors and AT1 blockers also lower the filtration of macromolecules across the glomerular barrier (Ma and Fogo 2001). In mice lacking ACE2, there may be changes in glucose tolerance, a reduction in first-phase insulin production, and a possible function for ACE2 in the onset of diabetes (Niu et al. 2008). Diabetes and COVID-19 interact with one another in a reciprocal manner. Diabetes increases a person’s susceptibility to COVID-19, and SARS-CoV-2 infection can exacerbate dysglycemia, inflammatory responses, and diabetic complications such as diabetic ketoacidosis (Pal and Bhadada 2020) and hypokalemia, which raise the likelihood of developing a life-threatening illness. Diabetes of both kinds I and II is linked to higher plasminogen levels, which has been theorized to make SARS CoV-2 more virulent (Fig. 2). Conversely, obesity common comorbidity of diabetes reduces systemic chronic inflammation by impacting the both innate and adaptive immune systems as well as the level of IL-6 and even TNF-α (Coelho et al. 2013). Diabetes and obesity have been linked to cytokine storm induction (Papadokostaki et al. 2020; Kaye et al. 2012), as well as coagulation system and thrombotic processes impairment, which are also identified in COVID-19 (Magro et al. 2020). Due to the glycosylation process, the expression of ACE2 is downregulated in diabetics, which may be a contributory factor for the severity of lung complications in comorbidity with SARS-CoV-2 invasion (Pal and Bhansali 2020). Overexpression of ACE2 is caused by the use of ACEis and ARB. When ACE2 is overexpressed, it can break down Ang II and produce angiotensin 1–7, which has beneficial effects on COVID-19 (anti-fibrotic, anti-oxidant, anti-inflammatory, and vasodilatory). DPP4 inhibitors influence the activity of fibroblasts and myofibroblasts to have anti-fibrotic effects while also having anti-inflammatory effects in the lung. The lower rate of lung injury and mortality in COVID-19 may be a result of these effects.
Effect of ACE2 on cardiovascular disease
According to the cardiovascular continuum, vascular disease, tissue damage, pathological remodeling, hypertension, target organ dysfunction, organ failure, and mortality are all risk factors for cardiovascular disease development. The preclinical cardiovascular disease manifests as hypertension, dyslipidemia, and diabetes in its early stages. An essential part of the pathogenesis of CVD (cardiovascular disease) is played by the renin-angiotensin system, and RAS blocking is a key component of the therapeutic approach for the management of CVD. It is now recognized that there is a kind of RAS in which ACE2 degrades Ang II, the primary effectors of the traditional RAS, and generates Ang (1–7). Through vasoconstriction and salt-water retention, Ang II raises blood pressure and promotes cardiac remodeling, fibrosis, inflammation, thrombosis, and plaque rupture. Numerous pieces of evidence show that ACE2 is essential for maintaining cardiovascular homeostasis. In addition, ACE2 is elevated in failing human hearts and atherosclerotic arteries, and its altered expression of cardiac and vascular disease in experimental models of CVD, and ACE2 is increased in failing human hearts and atherosclerotic vessels. ACE plays physiological functions through Ang II and AT1 receptors. Through the development of non-classical Ang (1–7) and Mas receptors, ACE2 acts the opposite functions to the ACE/Ang II/ATR1 axis (Santos et al. 2013; Burns 2007). The vasodilating and antiproliferative effects of this peptide are mediated via the binding of Ang (1–7) to the Mas receptor (Santos et al. 2003). The ACE2-Ang-(1–7) axis has been proven to be a unique RAS protective arm. A protective mechanism against renal injury may be reflected by increased ACE2 expression, principally by tipping the scales in favor of vasoprotective Ang (1–7) from Ang II. According to studies, loss of ACE2 can prevent the evolution of heart failure, while an increase in ACE2 expression can delay or even reverse the phenotype of heart failure. Patients with heart failure also have much higher circulating ACE2 activity than healthy individuals. The enhanced ACE2 activity in individuals with heart failure may be caused by the membrane-bound ACE2 being shed (Fig. 2).
ACE2 is a versatile enzyme with a variety of biological substrates (Fig. 2). It functions as a monocarboxypeptidase and cleaves a number of different non-RAAS peptides, including (des-Arg9)-bradykinin, a component of the kininogen-kinin system, which is involved in regulating cardiovascular homeostasis (Turner et al. 2002). In patients with COVID-19 precursors SARS (8%) and MERS (30%), CVD was one of the most prevalent co-morbidities. Intestinal epithelium, vascular endothelium, and the heart and lung all express ACE2, which provides a mechanism for the multi-organ dysfunction that can be seen with SARS-CoV-2 infection (Tikellis and Thomas 2012; Zhang et al. 2020b). There is mounting evidence that COVID-19 is associated with higher cardiovascular disease morbidity and death (CVD). The presence of ACE2 receptors on cardiac muscle cells may contribute to the high risk of COVID-19 in individuals with preexisting cardiovascular disease, indicating a potential role for the cardiovascular system in SARS-CoV-2 infection (Fig. 2). There is a significant risk of serious coronary infections in patients with CVD. Additionally, a higher prevalence of inflammatory cytokines is associated with COVID-19 instances and mediates atherosclerosis, procoagulant activation, and hemodynamic instability that result in ischemia and thrombosis (Bonow et al. 2020). Additionally, a higher prevalence of inflammatory cytokines is associated with COVID-19 instances and mediates atherosclerosis, procoagulant activation, and hemodynamic instability that result in ischemia and thrombosis (Bonow et al. 2020).
Conclusions
Compared to female patients with COVID-19, male patients had a much higher chance of dying. In contrast to patients who were older than 50, patients fewer than 50 had a much higher risk of dying. Patients with cancer, diabetes, kidney disease, pulmonary illness, cerebrovascular disease, hypertension, and other diseases had a significantly increased mortality rate. The risk of death linked with COVID-19 may be greatly decreased by implementing proper protection and interventions for COVID-19 patients in general, and in particular male patients with age 50 years who have comorbidities. The COVID-19 symptoms resemble those of the flu (e.g., fever, cough, or fatigue). A year with a high prevalence of respiratory illnesses brought on by the flu, respiratory syncytial virus, and other respiratory viruses saw COVID-19 outbreaks. There are significant effects of COVID-19 on pregnancies and ACE2 expression in the placenta as well as in maternal and fetal circulations. In addition, there are a number of potential therapeutic strategies that can cure pregnancy difficulties brought on by COVID-19 infections and inhibit the binding of ACE2 and SARS-CoV-2 by employing recombinant ACE2 or by blocking the viral S-protein RBD (AzinheiraNobrega Cruz et al. 2021). Pregnant women were more likely to seek hospitalization among reproductive women (15–44 years old) who tested positive for COVID-19 than nonpregnant women. Compared to pregnant women without the condition or even nonpregnant women with COVID-19, admission to the ICU was more common in pregnant women diagnosed with COVID-19 (Allotey et al. 2020; Ellington et al. 2020). Aging, diabetes, and obesity are the three primary risk factors for disease severity in pregnant women (AzinheiraNobrega Cruz et al. 2021; Ahlberg et al. 2020). The fact that older people are at higher risk of contracting COVID-19 infection may be related to their deteriorating immune systems, T-cell control, and declining CD4 counts (Liu et al. 2020). Knowing these indicators will help identify COVID-19 individuals who are more at risk and will enable a more focused and targeted strategy for preventing those fatalities. Success in vaccination efforts may derive from modifications in spike protein structure during receptor-mediated host cell entrance and further prediction of post-fusion events (Zemlin and Wiese 2020; Banerjee et al. 2020).
References
Ahlberg M, Neovius M, Saltvedt S, Soderling J, Pettersson K, Brandkvist C et al (2020) Association of SARS-CoV-2 test status and pregnancy outcomes. JAMA 324(17):1782–1785. https://doi.org/10.1001/jama.2020.19124
Aksoy H, Karadag AS, Wollina U (2020) Angiotensin II receptors: impact for COVID-19 severity. Dermatol Ther 33(6):e13989. https://doi.org/10.1111/dth.13989. Epub 2020 Jul 27. PMID: 32645228; PMCID: PMC7361069
Alenina N, Xu P, Rentzsch B, Patkin EL, Bader M (2008) Genetically altered animal models for Mas and angiotensin-(1–7). Exp Physiol 93(5):528–537. https://doi.org/10.1113/expphysiol.2007.040345. Epub 2007 Dec 21 PMID: 18156169
Allen AM, Zhuo J, Mendelsohn FA (2000) Localization and function of angiotensin AT1 receptors. Am J Hypertens 13(1 Pt 2):31S-38S. https://doi.org/10.1016/s0895-7061(99)00249-6. PMID: 10678286
Allotey J, Stallings E, Bonet M, Yap M, Chatterjee S, Kew T et al (2020) Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ 370, m3320. https://doi.org/10.1136/bmj.m3320
Arendse LB, Danser AHJ, Poglitsch M, Touyz RM, Burnett JC Jr, Llorens-Cortes C, Ehlers MR, Sturrock ED (2019) Novel therapeutic approaches targeting the renin-angiotensin system and associated peptides in hypertension and heart failure. Pharmacol Rev 71(4):539–570. https://doi.org/10.1124/pr.118.017129. PMID:31537750; PMCID:PMC6782023
AzinheiraNobrega Cruz N, Stoll D, Casarini DE, Bertagnolli M (2021) Role of ACE2 in pregnancy and potential implications for COVID-19 susceptibility. Clin Sci (lond) 135(15):1805–1824. https://doi.org/10.1042/CS20210284. PMID:34338772;PMCID:PMC8329853
Bader M, Ganten D (2008) Update on tissue renin-angiotensin systems. J Mol Med (berl) 86(6):615–621. https://doi.org/10.1007/s00109-008-0336-0. Epub 2008 Apr 15 PMID: 18414822
Banerjee A, Santra D, Maiti S (2020) Energetics and IC50 based epitope screening in SARS CoV-2 (COVID 19) spike protein by immunoinformatic analysis implicating for a suitable vaccine development. J Transl Med 18(1):281. https://doi.org/10.1186/s12967-020-02435-4. PMID:32650788;PMCID:PMC7351549
Batlle D, Wysocki J, Satchell K (2020) Soluble angiotensin-converting enzyme 2: a potential approach for coronavirus infection therapy? Clin Sci (lond) 134(5):543–545. https://doi.org/10.1042/CS20200163. PMID: 32167153
Berbudi A, Rahmadika N, Tjahjadi AI, Ruslami R (2020) Type 2 diabetes and its impact on the immune system. Curr Diabetes Rev 16(5):442–449. https://doi.org/10.2174/1573399815666191024085838. PMID:31657690; PMCID:PMC7475801
Bernardi S, Toffoli B, Zennaro C, Tikellis C, Monticone S, Losurdo P, Bellini G, Thomas MC, Fallo F, Veglio F, Johnston CI, Fabris B (2012) High-salt diet increases glomerular ACE/ACE2 ratio leading to oxidative stress and kidney damage. Nephrol Dial Transplant 27(5):1793–1800. https://doi.org/10.1093/ndt/gfr600. Epub 2011 Oct 29 PMID: 22036945
Bonow RO, Fonarow GC, O’Gara PT, Yancy CW (2020) Association of coronavirus disease 2019 (COVID-19) with myocardial injury and mortality. JAMA Cardiol 5(7):751–753. https://doi.org/10.1001/jamacardio.2020.1105. PMID: 32219362
Brunner HR (2001) Experimental and clinical evidence that angiotensin II is an independent risk factor for cardiovascular disease. Am J Cardiol 87(8A):3C-9C. https://doi.org/10.1016/s0002-9149(01)01538-7. PMID: 11334762
Burns KD (2007) The emerging role of angiotensin-converting enzyme-2 in the kidney. CurrOpin Nephrol Hypertens 16(2):116–121. https://doi.org/10.1097/MNH.0b013e3280123c0e. PMID: 17293686
CDC (2020) Coronavirus (COVID-19): symptoms of coronavirus. Centers for Disease Control and Prevention. Accessed 18 Apr 2020
Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L (2020) Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 395(10223):507–513. https://doi.org/10.1016/S0140-6736(20)30211-7. Epub 2020 Jan 30. PMID: 32007143; PMCID: PMC7135076
Coelho M, Oliveira T, Fernandes R (2013) Biochemistry of adipose tissue: an endocrine organ. Arch Med Sci. 2013 Apr 20;9(2):191–200. https://doi.org/10.5114/aoms.2013.33181. Epub 2013 Feb 10. PMID: 23671428; PMCID: PMC3648822
Crackower MA, Sarao R, Oudit GY, Yagil C, Kozieradzki I, Scanga SE, Oliveira-dos-Santos AJ, da Costa J, Zhang L, Pei Y, Scholey J, Ferrario CM, Manoukian AS, Chappell MC, BackxPH, Yagil Y, Penninger JM (2002) Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature 417(6891):822–8. https://doi.org/10.1038/nature00786. PMID: 12075344
Davern PJ, Head GA (2007) Fos-related antigen immunoreactivity after acute and chronic angiotensin II-induced hypertension in the rabbit brain. Hypertension 49(5):1170–1177. https://doi.org/10.1161/HYPERTENSIONAHA.106.086322. Epub 2007 Mar 5 PMID: 17339536
Donoghue M, Hsieh F, Baronas E, Godbout K, Gosselin M, Stagliano N, Donovan M, Woolf B, Robison K, Jeyaseelan R, Breitbart RE, Acton S (2000) A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1–9. Circ Res 87(5):E1-9. https://doi.org/10.1161/01.res.87.5.e1. PMID: 10969042
Ellington S, Strid P, Tong VT, Woodworth K, Galang R, Zambrano LD et al (2020) Characteristics of women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status — United States, January 22–June 7, 2020. MMWR Morb. Mortal. Wkly. Rep. 69(25):769–775. https://doi.org/10.15585/mmwr.mm6925a1
Fang L, Karakiulakis G, Roth M (2020) Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med 8(4):e21. https://doi.org/10.1016/S2213-2600(20)30116-8. Epub 2020 Mar 11. Erratum in: Lancet Respir Med (6):e54. PMID: 32171062; PMCID: PMC7118626
Ferguson AV, Bains JS (1997) Actions of angiotensin in the subfornical organ and area postrema: implications for long term control of autonomic output. Clin Exp Pharmacol Physiol 24(1):96–101. https://doi.org/10.1111/j.1440-1681.1997.tb01790.x. PMID: 9043813
Ferrario CM (1998) Angiotension-(1–7) and antihypertensive mechanisms. J Nephrol (6):278–83. PMID:10048492
Ferrario CM, Trask AJ, Jessup JA (2005) Advances in biochemical and functional roles of angiotensin-converting enzyme 2 and angiotensin-(1–7) in regulation of cardiovascular function. Am J Physiol Heart Circ Physiol 289(6):H2281–90. https://doi.org/10.1152/ajpheart.00618.2005. Epub 2005 Jul 29. PMID: 16055515; PMCID: PMC7203566
Ghazi L, Drawz P (2017) Advances in understanding the renin-angiotensin-aldosterone system (RAAS) in blood pressure control and recent pivotal trials of RAAS blockade in heart failure and diabetic nephropathy. F1000Res 21;6:F1000 Faculty Rev-297. https://doi.org/10.12688/f1000research.9692.1. PMID: 28413612; PMCID: PMC5365219
Givertz MM (2001) Manipulation of the renin-angiotensin system. Circulation 104(5):E14–E18. https://doi.org/10.1161/hc3001.094733. PMID: 11479264
Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, Cereda D, Coluccello A, Foti G, Fumagalli R, Iotti G, Latronico N, Lorini L, Merler S, Natalini G, Piatti A, Ranieri MV, Scandroglio AM, Storti E, Cecconi M, Pesenti A (2020) COVID-19 Lombardy ICU Network. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 28;323(16):1574–1581. https://doi.org/10.1001/jama.2020.5394. PMID: 32250385; PMCID: PMC7136855
Grundy SM, Benjamin IJ, Burke GL, Chait A, Eckel RH, Howard BV, Mitch W, Smith SC Jr, Sowers JR (1999) Diabetes and cardiovascular disease: a statement for healthcare professionals from the American Heart Association. Circulation 100(10):1134–1146. https://doi.org/10.1161/01.cir.100.10.1134.Erratum.In:Circulation2000Apr4;101(13):1629-31. PMID: 10477542
Guan WJ, Liang WH, Zhao Y, Liang HR, Chen ZS, Li YM, Liu XQ, Chen RC, Tang CL, Wang T, Ou CQ, Li L, Chen PY, Sang L, Wang W, Li JF, Li CC, Ou LM, Cheng B, Xiong S, Ni ZY, Xiang J, Hu Y, Liu L, Shan H, Lei CL, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Cheng LL, Ye F, Li SY, Zheng JP, Zhang NF, Zhong NS, He JX (2020) China Medical Treatment Expert Group for COVID-19. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J 55(5):2000547. https://doi.org/10.1183/13993003.00547-2020. PMID: 32217650; PMCID: PMC7098485
Hashimoto T, Perlot T, Rehman A, Trichereau J, Ishiguro H, Paolino M, Sigl V, Hanada T, Hanada R, Lipinski S, Wild B, Camargo SM, Singer D, Richter A, Kuba K, Fukamizu A, Schreiber S, Clevers H, Verrey F, Rosenstiel P, Penninger JM (2012) ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature 487(7408):477–481. https://doi.org/10.1038/nature11228. PMID:22837003; PMCID:PMC7095315
He Y, Yang W, Liu S, Gan L, Zhang F, Mu C, Wang J, Qu L, Wang R, Deng J, Ye Q, Yang X, Dong Y, Wang Q, Wei C, Hou Z, Yang L (2017) Interactions between angiotensin-converting enzyme-2 polymorphisms and high salt intake increase the risk of hypertension in the Chinese Wa population. Int J Clin Exp Pathol 10(11):11159–11168. PMID: 31966466; PMCID: PMC6965882
Hollenberg NK (2010) Direct renin inhibition and the kidney. Nat Rev Nephrol 6(1):49–55. https://doi.org/10.1038/nrneph.2009.201. Epub 2009 Nov 24 PMID: 19935744
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395(10223):497–506. https://doi.org/10.1016/S0140-6736(20)30183-5. Epub 2020 Jan 24. Erratum in: Lancet. 2020 Jan 30;: PMID: 31986264; PMCID: PMC7159299
Kaye SM, Pietiläinen KH, Kotronen A, Joutsi-Korhonen L, Kaprio J, Yki-Järvinen H, Silveira A, Hamsten A, Lassila R, Rissanen A (2012) Obesity-related derangements of coagulation and fibrinolysis: a study of obesity-discordant monozygotic twin pairs. Obesity (silver Spring) 20(1):88–94. https://doi.org/10.1038/oby.2011.287. Epub 2011 Sep 29 PMID: 21959347
Li J, Wang X, Chen J, Zhang H, Deng A (2020) Association of renin-angiotensin system inhibitors with severity or risk of death in patients with hypertension hospitalized for coronavirus disease 2019 (COVID-19) infection in Wuhan, China. JAMA Cardiol. https://doi.org/10.1001/jamacardio.2020.1624. Published online April 23, 2020.
Li B, Yang J, Zhao F, Zhi L, Wang X, Liu L, Bi Z, Zhao Y (2020) Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol 109(5):531–538. https://doi.org/10.1007/s00392-020-01626-9. Epub 2020 Mar 11. PMID: 32161990; PMCID: PMC7087935
Li XC, Zhang J, Zhuo JL (2017) The vasoprotective axes of the renin-angiotensin system: physiological relevance and therapeutic implications in cardiovascular, hypertensive and kidney diseases. Pharmacol Res 125(Pt A):21–38. https://doi.org/10.1016/j.phrs.2017.06.005. Epub 2017 Jun 12. PMID: 28619367; PMCID: PMC5607101
Liu PP, Blet A, Smyth D, Li H (2020) The science underlying COVID-19: implications for the cardiovascular system. Circulation. Epub ahead of print 15 April 2020. https://doi.org/10.1161/CIRCULATIONAHA.120.047549
Ma L, Fogo AB (2001) Role of angiotensin II in glomerular injury. Semin Nephrol 21(6):544–553. https://doi.org/10.1053/snep.2001.26793. PMID: 11709802
Magro C, Mulvey JJ, Berlin D, Nuovo G, Salvatore S, Harp J, Baxter-Stoltzfus A, Laurence J (2020) Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res 220:1–13. https://doi.org/10.1016/j.trsl.2020.04.007. Epub 2020 Apr 15. PMID:32299776; PMCID: PMC7158248
Masi S, Uliana M, Virdis A (2019) Angiotensin II and vascular damage in hypertension: role of oxidative stress and sympathetic activation. VasculPharmacol 115:13–17. https://doi.org/10.1016/j.vph.2019.01.004. Epub 2019 Jan 30 PMID: 30707954
McKinley MJ, Allen AM, Burns P, Colvill LM, Oldfield BJ (1998) Interaction of circulating hormones with the brain: the roles of the subfornical organ and the organum vasculosum of the lamina terminalis. Clin Exp Pharmacol Physiol Suppl 25:S61–S67. https://doi.org/10.1111/j.1440-1681.1998.tb02303.x. PMID: 980919
Monteil V, Kwon H, Prado P, Hagelkrüys A, Wimmer RA, Stahl M, Leopoldi A, Garreta E, Hurtado Del Pozo C, Prosper F, Romero JP, Wirnsberger G, Zhang H, Slutsky AS, Conder R, Montserrat N, Mirazimi A, Penninger JM (2020) Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell 14;181(4):905–913.e7. https://doi.org/10.1016/j.cell.2020.04.004. Epub 2020 Apr 24. PMID: 32333836; PMCID: PMC7181998
Niu MJ, Yang JK, Lin SS, Ji XJ, Guo LM (2008) Loss of angiotensin-converting enzyme 2 leads to impaired glucose homeostasis in mice. Endocrine 34(1–3):56–61. https://doi.org/10.1007/s12020-008-9110-x. Epub 2008 Oct 28. PMID: 18956256
Novel Coronavirus Pneumonia Emergency Response Epidemiology Team (2020) Vital surveillances: the epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) – China, 2020. China CDC Weekly 2:113–122
Obukhov AG, Stevens BR, Prasad R, Li Calzi S, Boulton ME, Raizada MK, Oudit GY, Grant MB (2020) SARS-CoV-2 Infections and ACE2: clinical outcomes linked with increased morbidity and mortality in individuals with diabetes. Diabetes 69(9):1875–1886. https://doi.org/10.2337/dbi20-0019. Epub 2020 Jul 15. PMID: 32669391; PMCID: PMC745803
Oudit GY, Kassiri Z, Jiang C, Liu PP, Poutanen SM, Penninger JM, Butany J (2009) SARS-coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. Eur J Clin Invest 39(7):618–625. https://doi.org/10.1111/j.1365-2362.2009.02153.x. Epub 2009 May 6. PMID: 19453 650; PMCID: PMC71 63766
Pal R, Bhansali A (2020) COVID-19, diabetes mellitus and ACE2: the conundrum. Diabetes Res Clin Pract 162:108132 https://doi.org/10.1016/j.diabres.2020.108132. Epub. PMID: 32234504; PMCID:PMC7118535
Pal R, Bhadada SK (2020) COVID-19 and diabetes mellitus: an unholy interaction of two pandemics. Diabetes MetabSyndr 14(4):513–517. https://doi.org/10.1016/j.dsx.2020.04.049. Epub 2020 May 6. PMID: 32388331; PMCID: PMC7202837
Papadokostaki E, Tentolouris N, Liberopoulos E (2020) COVID-19 and diabetes: what does the clinician need to know? Prim Care Diabetes 14(5):558–563. https://doi.org/10.1016/j.pcd.2020.06.010. Epub 2020 Jul 3. PMID: 32654982; PMCID: PMC7332931
Paudel SS (2020) A meta-analysis of 2019 novel coronavirus patient clinical characteristics and comorbidities. Res Sq. https://doi.org/10.21203/rs.3.rs-21831/v1. Accessed 18 Apr 2020
Raizada MK, Ferreira AJ (2007) ACE2: a new target for cardiovascular disease therapeutics. J Cardiovasc Pharmacol 50(2):112–119. https://doi.org/10.1097/FJC.0b013e3180986219. PMID: 17703127
Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, the Northwell COVID-19 Research Consortium, Barnaby DP, Becker LB, Chelico JD, Cohen SL, Cookingham J, Coppa K, Diefenbach MA, Dominello AJ, Duer-Hefele J, Falzon L, Gitlin J, Hajizadeh N, Harvin TG, Hirschwerk DA, Kim EJ, Kozel ZM, Marrast LM, Mogavero JN, Osorio GA, Qiu M, Zanos TP (2020) Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA 323(20):2052–2059. https://doi.org/10.1001/jama.2020.6775. Erratum in: JAMA. 2020 May 26;323(20):2098. PMID: 32320003; PMCID: PMC7177629
Roglic G, Unwin N, Bennett PH, Mathers C, Tuomilehto J, Nag S, Connolly V, King H (2005) The burden of mortality attributable to diabetes: realistic estimates for the year 2000. Diabetes Care 28(9):2130–2135. https://doi.org/10.2337/diacare.28.9.2130. PMID:16123478
Sampaio WO, Souza dos Santos RA, Faria-Silva R, da Mata Machado LT, Schiffrin EL, Touyz RM (2007) Angiotensin-(1–7) through receptor Mas mediates endothelial nitric oxide synthase activation via Akt-dependent pathways. Hypertension (1):185–92. https://doi.org/10.1161/01.HYP.0000251865.35728.2f. Epub 2006 Nov 20. PMID: 17116756
Santos RA, Ferreira AJ, Verano-Braga T, Bader M (2013) Angiotensin-converting enzyme 2, angiotensin-(1–7) and Mas: new players of the renin-angiotensin system. J Endocrinol 216(2):R1–R17. https://doi.org/10.1530/JOE-12-0341. PMID: 23092879
Santos RA, Simoes e Silva AC, Maric C, Silva DM, Machado RP, de Buhr I, Heringer-Walther S, Pinheiro SV, Lopes MT, Bader M, Mendes EP, Lemos VS, Campagnole-Santos MJ, Schultheiss HP, Speth R, Walther T (2003) Angiotensin-(1–7) is an endogenous ligand for the G protein-coupled receptor Mas. Proc Natl Acad Sci USA 100(14):8258–63. https://doi.org/10.1073/pnas.1432869100. Epub 2003 Jun 26. PMID: 12829792; PMCID: PMC166216
Shang J, Ye G, Shi K, Wan Y, Luo C, Aihara H, Geng Q, Auerbach A, Li F (2020) Structural basis of receptor recognition by SARS-CoV-2. Nature 581(7807):221–224. https://doi.org/10.1038/s41586-020-2179-y. Epub PMID: 32225175; PMCID: PMC7328981
Song Z, Xu Y, Bao L, Zhang L, Yu P, Qu Y, Zhu H, Zhao W, Han Y, Qin C (2019) From SARS to MERS, thrusting coronaviruses into the spotlight. Viruses 11(1):59. https://doi.org/10.3390/v11010059. PMID:30646565;PMCID:PMC6357155
South AM, Shaltout HA, Washburn LK, Hendricks AS, Diz DI, Chappell MC (2019) Fetal programming and the angiotensin-(1–7) axis: a review of the experimental and clinical data. Clin Sci (lond) 133(1):55–74. https://doi.org/10.1042/CS20171550. PMID:30622158;PMCID:PMC6716381
Thatcher SE, Gupte M, Hatch N, Cassis LA (2012) Deficiency of ACE2 in bone-marrow-derived cells increases expression of TNF-α in adipose stromal cells and augments glucose intolerance in obese C57BL/6 mice. Int J Hypertens. 2012;2012:762094. https://doi.org/10.1155/2012/762094. Epub PMID: 22518292; PMCID: PMC3296206
Tikellis C, Bernardi S, Burns WC (2011) Angiotensin-converting enzyme 2 is a key modulator of the renin-angiotensin system in cardiovascular and renal disease. CurrOpin Nephrol Hypertens 20(1):62–68. https://doi.org/10.1097/MNH.0b013e328341164a. PMID: 21099686
Tikellis C, Thomas MC (2012) Angiotensin-converting enzyme 2 (ACE2) is a key modulator of the renin angiotensin system in health and disease. Int J Pept 256294. https://doi.org/10.1155/2012/256294. Epub 2012 Mar 20. PMID: 22536270; PMCID: PMC3321295
Tipnis SR, Hooper NM, Hyde R, Karran E, Christie G, Turner AJ (2000) A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J Biol Chem 275(43):33238–43. https://doi.org/10.1074/jbc.M002615200. PMID: 10924499
Towler P, Staker B, Prasad SG, Menon S, Tang J, Parsons T, Ryan D, Fisher M, Williams D, Dales NA, Patane MA, Pantoliano MW (2004) ACE2 X-ray structures reveal a large hinge-bending motion important for inhibitor binding and catalysis. J Biol Chem 23;279(17):17996–8007. https://doi.org/10.1074/jbc.M311191200. Epub 2004 Jan 30. PMID: 14754895; PMCID: PMC7980034
Turner AJ (2015) Chapter 25:ACE2 cell biology , regulation, and physiological functions. In Unger T, Ulrike M, Steckelings UM, dos Santos RA (eds). The protective arm of rennin angiotensin system (RAS): functional aspects and therapeutic implications. AcademicPress. 2015; pp.185–189. https://doi.org/10.1016/B978-0-12-801364-9.00025-0
Turner AJ, Hiscox JA, Hooper NM (2004) ACE2: from vasopeptidase to SARS virus receptor. Trends Pharmacol Sci 25(6):291–294. https://doi.org/10.1016/j.tips.2004.04.001. PMID:15165741;PMCID:PMC7119032
Turner AJ, Tipnis SR, Guy JL, Rice G, Hooper NM (2002) ACEH/ACE2 is a novel mammalian metallocarboxypeptidase and a homologue of angiotensin-converting enzyme insensitive to ACE inhibitors. Can J Physiol Pharmacol 80(4):346–353. https://doi.org/10.1139/y02-021. PMID: 12025971
Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA, Solomon SD (2020) Renin-angiotensin-aldosterone system inhibitors in patients with Covid-19. N Engl J Med 382(17):1653–1659. Epub 2020 Mar 30. PMID: 32227760; PMCID: PMC7121452
Vickers C, Hales P, Kaushik V, Dick L, Gavin J, Tang J, Godbout K, Parsons T, Baronas E, Hsieh F, Acton S, Patane M, Nichols A, Tummino P (2002) Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J Biol Chem 277(17):14838–14843. https://doi.org/10.1074/jbc.M200581200. Epub 2002 Jan 28 PMID: 11815627
Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z (2020) Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan. China JAMA 323(11):1061–1069. https://doi.org/10.1001/jama.2020.1585. Erratum.In:JAMA.2021Mar16;325(11):1113.PMID:32031570;PMCID:PMC7042881
Wang Y, Wang Y, Luo W, Huang L, Xiao J, Li F, Qin S, Song X, Wu Y, Zeng Q, Jin F, Wang Y (2020) A comprehensive investigation of the mRNA and protein level of ACE2, the putative receptor of SARS-CoV-2, in human tissues and blood cells. Int J Med Sci 17(11):1522–1531. https://doi.org/10.7150/ijms.46695. PMID:32669955; PMCID:PMC7359402
Wysocki J, Garcia-Halpin L, Ye M, Maier C, Sowers K, Burns KD, Batlle D (2013) Regulation of urinary ACE2 in diabetic mice. Am J Physiol Renal Physiol 15;305(4):F600–11. https://doi.org/10.1152/ajprenal.00600.2012. Epub 2013 Jun 12. PMID:23761674; PMCID: PMC3891267
Yan R, Zhang Y, Li Y, Xia L, Guo Y, Zhou Q (2020) Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science 367(6485):1444–1448. https://doi.org/10.1126/science.abb2762. Epub PMID: 32132184; PMCID: PMC7164635
Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, Wu Y, Zhang L, Yu Z, Fang M, Yu T, Wang Y, Pan S, Zou X, Yuan S, Shang Y (2020) Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med (5):475–481. https://doi.org/10.1016/S2213-2600(20)30079-5. Epub 2020 Feb 24. Erratum in: Lancet Respir Med (4):e26. PMID: 32105632; PMCID: PMC7102538
Ye M, Wysocki J, William J, Soler MJ, Cokic I, Batlle D (2006) Glomerular localization and expression of angiotensin-converting enzyme 2 and angiotensin-converting enzyme: implications for albuminuria in diabetes. J Am Soc Nephrol 17(11):3067–3075. https://doi.org/10.1681/ASN.2006050423. Epub 2006 Oct 4 PMID: 17021266
Zaman MA, Oparil S, Calhoun DA (2002) Drugs targeting the renin-angiotensin-aldosterone system. Nat Rev Drug Discov 1(8):621–636. https://doi.org/10.1038/nrd873. PMID: 12402502
Zemlin AE, Wiese OJ (2020) Coronavirus disease 2019 (COVID-19) and the renin-angiotensin system: a closer look at angiotensin-converting enzyme 2 (ACE2). Ann Clin Biochem 57(5):339–350. https://doi.org/10.1177/0004563220928361. Epub 2020 Jun 2. PMID: 32369402; PMCID: PMC7267743
Zhang JJ, Dong X, Cao YY, Yuan YD, Yang YB, Yan YQ, Akdis CA, Gao YD (2020a) Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan. China Allergy 75(7):1730–1741. https://doi.org/10.1111/all.14238. Epub 2020 Feb 27 PMID: 32077115
Zhang H, Penninger JM, Li Y, Zhong N, Slutsky AS (2020b) Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med 46(4):586–590. https://doi.org/10.1007/s00134-020-05985-9. Epub 2020 Mar 3. PMID: 32125455; PMCID: PMC7079879
Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B (2020) Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395(10229):1054–1062. https://doi.org/10.1016/S0140-6736(20)30566-3. Epub 2020 Mar 11. Erratum in: Lancet 395(10229):1038. Erratum in: Lancet. 2020 Mar 28;395(10229):1038. PMID: 32171076; PMCID: PMC7270627
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was not supported by any funding.
Conflict of interest
The authors declare no competing interests.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Informed consent
Received.
Consent for publication
Received.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Santra, D., Banerjee, A., De, S.K. et al. Relation of ACE2 with co-morbidity factors in SARS-CoV-2 pathogenicity. Comp Clin Pathol 32, 179–189 (2023). https://doi.org/10.1007/s00580-023-03434-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00580-023-03434-9