Abstract
Purpose
In many countries, patients are generally allowed to have clear fluids until 2–3 h before surgery. In Japan, long preoperative fasting is still common practice. To shorten the preoperative fasting period in Japan, we tested the safety and efficacy of oral rehydration therapy until 2 h before surgery.
Methods
Three hundred low-risk patients scheduled for morning surgery in six university-affiliated hospitals were randomly assigned to an oral rehydration solution (ORS) group or to a fasting group. Patients in the ORS group consumed up to 1,000 ml of ORS containing balanced glucose and electrolytes: 500 ml between 2100 the night before surgery and the time they woke up the next morning and 500 ml during the morning of surgery until 2 h before surgery. Patients in the fasting group started fasting at 2100 the night before surgery. Primary endpoints were gastric fluid volume and pH immediately after anesthesia induction. Several physiological measures of hydration and electrolytes including the fractional excretion of sodium (FENa) and the fractional excretion of urea nitrogen (FEUN) were also evaluated.
Results
Mean (SD) gastric fluid volume immediately after anesthesia induction was 15.1 (14.0) ml in the ORS group and 17.5 (23.2) ml in the fasting group (P = 0.30). The mean difference between the ORS group and fasting group was −2.5 ml. The 95% confidence interval ranged from −7.1 to +2.2 ml and did not include the noninferior limit of +8 ml. Mean (SD) gastric fluid pH was 2.1 (1.9) in the ORS group and 2.2 (2.0) in the fasting group (P = 0.59). In the ORS group, mean FENa and FEUN immediately after anesthesia induction were both significantly greater than those in the fasting group (P < 0.001 for both variables). The ORS group reported they had been less thirsty and hungry before surgery (P < 0.001, 0.01).
Conclusions
Oral rehydration therapy until 2 h before surgery is safe and feasible in the low-risk Japanese surgical population. Physicians are encouraged to use this practice to maintain the amount of water in the body and electrolytes and to improve the patient’s comfort.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Long preoperative fasting has been a standard practice for patients undergoing elective surgery to avoid vomiting and pulmonary aspiration during anesthesia induction. However, in the 1990s, physicians and scientists in Western countries started to question whether long preoperative fasting might be increasing the incidence of dehydration and perioperative complications. In 1999, the American Society of Anesthesiologists (ASA) issued a practice guideline [1] for preoperative fasting in which patients (except those with delayed gastric emptying) are allowed to have clear fluids (water, fruit juices without pulp, carbonated beverages, clear tea, and black coffee) until 2 h before surgery. European anesthesia societies have established similar guidelines [2–4]. A survey of 258 medical institutions in five northern European countries revealed that most of them allowed clear fluids up to a median of 2 h before surgery [5]. In contrast, a 2003 nationwide survey of preoperative fasting in Japanese anesthesia-teaching hospitals revealed that most hospitals used long preoperative fasting: the median duration of fasting in adults was 9 h before morning surgery and 6 h before afternoon surgery [6].
Perioperative fluid and electrolyte balance should be managed so that patients enter the operating room with normal fluid and electrolyte balance, instead of starting the procedure at anesthesia induction [7]. For surgical patients, recent Japanese studies have shown that oral rehydration therapy is a safe and effective method for managing preoperative water and electrolyte intake [8], but this result still needs to be confirmed by larger randomized clinical trials.
Therefore, we conducted a multicenter clinical study to investigate the safety and efficacy of oral rehydration therapy until 2 h before surgery by using an oral rehydration solution (ORS). The objective of our study was to improve current preoperative fasting practice and to evaluate the efficacy of preoperative oral rehydration therapy.
Patients and methods
The study was conducted at six institutions: Tokai University Hospital, Saitama Medical University Saitama Medical Center, Jichi Medical University Saitama Medical Center, Nippon Medical School Hospital, Kyorin University Hospital, and Kawasaki Medical School Hospital. The study was approved by the Institutional Ethics Committee of each institution and was conducted in accordance with Declaration of Helsinki-Ethical Principles for Medical Research Involving Human Subjects and with the Ethical Guidelines for Clinical Research issued by Ministry of Health, Labour, and Welfare.
Patients
Patients were eligible for the study if they were between 20 and 80 years old; scheduled to have elective, morning surgery requiring general anesthesia between January 2010 and March 2011; and were ASA physical status I or II. Exclusion criteria were dysphagia, esophagostenosis, the inability to take food orally as a result of neurological and other disorders; gastrointestinal obstruction; renal dysfunction (a creatinine clearance of 20 ml min−1 or lower or dialysis); electrolyte abnormalities; body mass index (BMI) of 35 kg m−2 or larger; and pregnancy. Also excluded were patients receiving intravenous fluid for any reasons preoperatively; a premedication; an H2 receptor blocker or a proton pump inhibitor; a diuretic; or steroids. Patients otherwise deemed unsuitable for the study were also excluded.
Experimental plan
Before the study, written informed consents were obtained after the patients were extensively informed about the study procedure. The enrolled patients were assigned at random to either an ORS group or to a fasting group at each institution. Randomization was performed according to a computer-generated schedule with a permuted-block design.
Primary endpoints were gastric volume and its pH immediately after anesthesia induction. Secondary endpoints included the incidence of vomiting and aspiration associated with anesthesia induction, the relationships of gastric fluid volume and with age and with BMI in the ORS group, the effect of rehydration, vital signs, and the patient’s comfort.
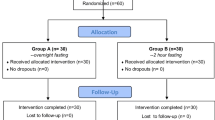
Patients in both groups were allowed to have food or drink until 2100 on the night before surgery (Fig. 1). Patients in the ORS group consumed up to 1,000 ml of ORS (OS-1; Otsuka Pharmaceutical Factory, Tokushima, Japan; Table 1): 500 ml between 2100 the night before surgery and the time they woke up the next morning and 500 ml in the morning of surgery up until 2 h before surgery. Patients in the fasting group started fasting at 2100 the night before surgery. Patients in either group who needed to take medications orally before surgery were allowed to do so with 20–50 ml water until 2 h before surgery. Types of surgery, anesthesia methods, and intraoperative fluid management were at the discretion of each institution.
Gastric fluid volume and gastric fluid pH of the patients were measured in supine position immediately after anesthesia induction. Any incidences of vomiting or aspiration were recorded. Patients were intubated by mouth or nose with a nasogastric tube (the tube was inserted until 60 cm of the tube was placed inside the body). Correct placement of the tube in the stomach was confirmed by hearing the air with a stethoscope, and gastric contents were suctioned at 60, 55, and 50 cm from the top of the catheter with a catheter syringe [9]. This procedure was repeated three times. Gastric fluid was sampled by the same person at each institution, whenever possible. Gastric fluid pH was measured by SRL (SRL, Inc., Clinical Laboratory Testing Company, Tokyo, Japan). We evaluated the efficacy of the ORS to provide hydration and electrolytes by measuring the fractional excretion of sodium (FENa), the fractional excretion of urea nitrogen (FEUN), ratio of serum urea nitrogen to serum creatinine, hematocrit, serum total protein, serum albumin, serum electrolytes (sodium, potassium, and chlorine), urine output (between 2100 the night before surgery and start of surgery and during surgery), and the amount of vasoconstrictors or intravenous solutions used during surgery. Blood was taken immediately after anesthesia induction, and the first urine sample was taken after a urinary catheter was placed. Patients were asked whether they were thirsty, hungry, or anxious before they entered the operating room. They were also asked twice whether they were nauseous or had vomited, on the night after surgery and at noon the next day. Patients completed the questionnaire form. Vital signs (blood pressure, pulse rate, and body temperature) were measured after dinner the night before surgery; before entering the operating room; at 5, 10, 15, 30, and 60 min after anesthetic induction; and every 1 h afterwards. Blood and urine samples were tested by SRL.
Statistical methods
In this noninferiority study, gastric volumes were compared between the ORS group and the fasting group. Sample size calculation was based on a Cochrane review of studies on preoperative fasting and assumed that between-group difference in the residual gastric volume was 0 ml, noninferiority margin was +8 ml, standard deviation of residual gastric volume was 25 ml, two-tailed significance level was 0.05, and power was 0.80 [10]. The needed sample size was 122 per group, which was increased to 150 per group to allow for withdrawals.
For the primary endpoint, mean, standard deviation, maximum, median, minimum, third quartile, and first quartile of the gastric fluid volume and gastric fluid pH were calculated for each group. For the secondary endpoints, mean standard deviation, maximum, median, minimum, third quartile, and first quartile of FENa and FEUN were calculated. For other results, mean and standard deviation were calculated for each group. The SAS statistical software package, version 9.1.3 (SAS Institute Japan, Tokyo, Japan), was used for statistical analyses. Differences were considered statistically significant at the 0.05 level for both unpaired t tests and Chi-square test. Differences in patient demographic characteristics were considered significant at the 0.15 level. Pearson product-moment correlation coefficient was used for correlation analyses. Analysis of variance was performed on gastric fluid volume and gastric fluid pH with institutions and groups used as factors to check for confounding.
Results
Of 300 patients enrolled, 150 were assigned to the ORS group and 150 to the fasting group. Of these, 26 withdrew, leaving 135 in the ORS group and 139 in the fasting group (Fig. 2). Patients underwent otorhinolaryngological surgery, orthopedic/plastic surgery, gynecological surgery, breast and thyroid surgery, or thoracic surgery. BMI was significantly different between groups, but the difference was not clinically important. Other demographic characteristics did not differ significantly between groups (Table 2). Mean (SD) ORS intake in the ORS group was 414 (137) ml the night before surgery and 398 (143) ml on the morning of surgery.
Mean (SD) gastric fluid volume at anesthesia induction did not differ significantly between groups (P = 0.30): 15.1 (14.0) ml (range, 0–60 ml) in the ORS group and 17.5 (23.2) ml (range, 0–155 ml) in the fasting group (Fig. 3). The mean difference between the ORS group and fasting group was −2.5 ml. The 95% confidence interval ranged from −7.1 to +2.2 ml and did not include the noninferior limit of +8 ml. Mean (SD) gastric fluid pH was 2.1 (1.9) in the ORS group and 2.2 (2.0) in the fasting group; there was no significant difference between groups (P = 0.59; Table 3). Analysis of variance revealed no confounding between institutions and groups on gastric fluid volume or gastric fluid pH (data not reported). No incidence of vomiting or aspiration was reported. A statistically significant but clinically unimportant inverse relationship was seen between age and gastric fluid volume in the ORS group (R 2 = 0.0809, P = 0.001); the older the patient, the smaller the gastric fluid volume was to become. There was no correlation between the BMI and gastric fluid volume (R 2 = 0.0003, P = 0.85).
In the ORS group, mean FENa and FEUN immediately after anesthesia induction were both significantly greater than those in the fasting group (P < 0.001 for both variables): mean (SD) FENa was 0.94% (0.45%) in the ORS group, 0.64% (0.41%) in the fasting group, and mean (SD) FEUN was 33.7% (8.2%) in the ORS group and 27.2% (9.2%) in the fasting group (Fig. 4). The mean (SD) ratio of serum urea nitrogen to serum creatinine was significantly (P < 0.01) smaller in the ORS group [17.9 (4.9)] than in the fasting group [19.7 (5.2)]. The urine output between the night before surgery and start of surgery and up to 1 h after surgery was significantly greater in the ORS group (P < 0.001, 0.01). None of the physiological characteristics or vital signs differed between groups (Table 3). Both thirst and hunger before surgery were reported by significantly fewer patients in the ORS group (P < 0.001, 0.01), although the proportion of those reporting perioperative anxiety, nausea, and vomiting did not differ significantly between groups (Table 4).
Fractional excretion of sodium (FENa) and fractional excretion of urea nitrogen (FEUN) immediately after anesthesia induction in patients who received an oral rehydration solution and those who fasted overnight. (Plots show medians, ranges, and interquartile ranges.) Statistical analysis: unpaired t test α = 0.05). FENa = (urine sodium × serum creatinine)/(serum sodium ×urine creatinine), FEUN = (urine urea nitrogen × serum creatinine)/(blood urea nitrogen × urine creatinine)
Discussion
In the present study, gastric fluid volume in the ORS group was not inferior to that in the fasting group. Others have reported that gastric fluid volume was smaller in patients who received clear fluids until 2–3 h before surgery than it was in fasting patients, and our study results showed the same trends [10]. If the gastric fluid volume is 200 ml or larger when anesthesia is induced, the patient is at increased risk for vomiting and aspiration [2]. However, no patient had a gastric volume greater than 200 ml: the maximum volume was 60 ml in the ORS group and 155 ml in the fasting group. Gastric fluid pH did not differ significantly between the groups. Thus, oral rehydration therapy is feasible with sufficient safety margin for up to 2 h before surgery. Perioperative fasting guidelines in adults and children issued by the European Society of Anaesthesiology state that patients can safely drink carbohydrate-rich fluids up to 2 h before surgery. However, the evidence for safety is derived from studies of products specifically developed for perioperative use (predominantly maltodextrins); not all carbohydrates are necessarily safe [4]. We also believe beverages with clearly established evidence of safety should be given to patients undergoing surgery.
We excluded patients aged 80 years or older, severely obese patients (BMI ≥ 35 kg m−2), and pregnant women. However, gastric fluid volume was slightly inversely related to age in the ORS group, and gastric fluid volume was not related to BMI. In studies with elderly patients, clear fluids intake until 2 h before surgery did not cause problems [11, 12]. In studies with obese patients (BMI > 30 kg m−2), the gastric fluid volume in patients who received clear fluids until 2 h before surgery was similar to that in fasting patients [13]. A study of obese pregnant women showed that the gastric fluid volume 1 h after they drank 300 ml water was the same as the volume when fasting before drinking water [14]. Practice Guidelines for Obstetric Anesthesia issued by the ASA also state that patients can safely receive clear fluids until 2 h before surgery, except those who at increased risk for operative delivery, such as a non-reassuring fetal heart rate pattern [15]. Therefore, it seems reasonable that oral rehydration therapy can be safely administered to elderly, obese, or pregnant patients up to 2 h before surgery.
Both FENa and FEUN, which we used to evaluate the adequacy of hydration and electrolytes provided by the ORS, indicate the percentages of sodium and urea nitrogen that are filtrated through the glomerulus and ultimately excreted to the urine; that is, they are used as an index for renal blood flow [16, 17]. Both parameters decrease in dehydrated patients with normal renal function. (We excluded patients with a creatinine clearance of 20 ml min−1 or less or those on dialysis.) Our results revealed both FENa and FEUN in the fasting group were significantly lower than those in the ORS group. Also, the ratio of serum urea nitrogen to serum creatinine was significantly higher, and urine output before surgery and during the first hour of surgery were significantly lower in the fasting group. Furthermore, before surgery, 65.0% in the fasting group reported being thirsty. Thus, fasting starting on the night before surgery can result in a decreased amount of water in the body, and the decrease would have been prevented with oral rehydration therapy. The amount of vasoconstrictors or intravenous solutions used during surgery did not differ significantly between groups. The following mechanisms can be proposed: there were many confounding factors including the type of surgery, methods of anesthesia, and type and administration rate of intravenous fluids. Although FENa, FEUN, and serum biochemical profiles suggested the positive effects of ORS, actual effect on the amount of water in the body could have been minimal.
In our study, the only apparent clinical advantages were that patients in the ORS group reported being less thirsty and hungry. However, patient comfort is among the important clinical indices in the era of fast-track surgery. The mean (SD) ORS intake between the night before surgery and 2 h before surgery was 812 (240) ml, which was larger than expected and indicates the ORS is well tolerated. Also, there were few incidences of nausea or vomiting associated with ORS intake after surgery.
The safety and efficacy of the ORS for mildly to moderately dehydrated patients have already been studied [18–20]. Our study provided further supporting data regarding the safety and efficacy of the ORS in preoperative patients.
Limitations of the study
We did not include high-risk patients, patients with ASA physical status III or higher, and the safety and efficacy of the ORS in these patients are still unknown. Also, the effects of the supplemental electrolytes and glucose cannot be differentiated from those of rehydration. Therefore, to further investigate the true benefits of oral rehydration with the ORS, another study should be undertaken with water as a control arm under the specified type of surgery and methods of anesthesia and intravenous administration maintenance.
Conclusions
We investigated the safety and efficacy of oral rehydration therapy until 2 h before surgery in a multicenter randomized controlled clinical study. Gastric volume in the ORS group immediately after anesthesia induction was not inferior to that in the fasting group, and no significant difference in pH was found between groups. Our study supports that oral rehydration therapy until 2 h before surgery is safe. Thus, healthcare professionals should also be encouraged to use oral rehydration therapy until 2 h before surgery to maintain the amount of water in the body and electrolytes and to improve the patient’s comfort.
References
Practice guidelines for preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration: application to healthy patients undergoing elective procedures: a report by the American Society of Anesthesiologist Task Force on Preoperative Fasting. Anesthesiology. 1999;90:896–905.
Ljungqvist O, Søreide E. Preoperative fasting. Br J Surg. 2003;90:400–6.
Søreide E, Eriksson LI, Hirlekar G, Eriksson H, Henneberg SW, Sandin R, Raeder J. Pre-operative fasting guidelines: an update. Acta Anaesthesiol Scand. 2005;49:1041–7.
Smith I, Kranke P, Murat I, Smith A, O’Sullivan G, Sreide E, Spies C, In’t Veld B. Perioperative fasting in adults and children: guidelines from the European Society of Anaesthesiology. Eur J Anaesthesiol. 2011;28:556–69.
Hannemann P, Lassen K, Hausel J, Nimmo S, Ljungqvist O, Nygren J, Soop M, Fearon K, Andersen J, Revhaug A, von Meyenfeldt MF, Dejong CH, Spies C. Patterns in current anaesthesiological peri-operative practice for colonic resections: a survey in five northern-European countries. Acta Anaesthesiol Scand. 2006;50:1152–60.
Shime N, Ono A, Chihara E, Tanaka Y. Current practice of preoperative fasting: a nationwide survey in Japanese anesthesia-teaching hospitals. J Anesth. 2005;19:187–92.
Lobo DN, Macafee DA, Allison SP. How perioperative fluid balance influences postoperative outcomes. Best Pract Res Clin Anaesthesiol. 2006;20:439–55.
Taniguchi H, Sasaki T, Fujita H, Takamori M, Kawasaki R, Momiyama Y, Takano O, Shibata T, Goto T. Preoperative fluid and electrolyte management with oral rehydration therapy. J Anesth. 2009;23:222–9.
Suzuki A, Kumano H, Osaka S, Shiomi Y, Moroi K, Ishimura N, Nishiwada M. The effects of preoperative drinking and H2 blocker on gastric acid secretion (in Japanese with English abstract). Masui (Jpn J Anesthesiol). 1996;45:445–8.
Brady M, Kinn S, Stuart P. Preoperative fasting for adults to prevent perioperative complications. Cochrane Database Syst Rev 2003;4:CD004423.
Rapp-Kesek D, Stridsberg M, Andersson LG, Berne C, Karlsson T. Insulin resistance after cardiopulmonary bypass in the elderly patient. Scand Cardiovasc J. 2007;41:102–8.
Protic A, Turina D, Matanić D, Spanjol J, Zuvic-Butorac M, Sustic A. Effect of preoperative feeding on gastric emptying following spinal anesthesia: a randomized controlled trial. Wien Klin Wochenschr. 2010;122:50–3.
Maltby JR, Pytka S, Watson NC, Cowan RA, Fick GH. Drinking 300 mL of clear fluid two hours before surgery has no effect on gastric fluid volume and pH in fasting and non-fasting obese patients. Can J Anaesth. 2004;51:111–5.
Wong CA, McCarthy RJ, Fitzgerald PC, Raikoff K, Avram MJ. Gastric emptying of water in obese pregnant women at term. Anesth Analg. 2007;105:751–5.
Practice guidelines for obstetric anesthesia: an updated report by the American Society of Anesthesiologists Task Force on Obstetric Anesthesia. Anesthesiology. 2007;106:843–63.
Irwin RS, Rippe JM. Irwin and Rippe’s intensive care medicine. 6th ed ed. Philadelphia: Lippincott Williams & Wilkins; 2008. p. 880–1.
Carvounis CP, Nisar S, Guro-Razuman S. Significance of the fractional excretion of urea in the differential diagnosis of acute renal failure. Kidney Int. 2002;62:2223–9.
Mauer AM, Dweck HS, Finberg L, Holmes F, Reynolds JW, Suskind RM, Woodruff CW, Hellerstein S. American Academy of Pediatrics Committee on Nutrition: Use of oral fluid therapy and posttreatment feeding following enteritis in children in a developed country. Pediatrics. 1985;75:358–61.
Avery ME, Snyder JD. Oral therapy for acute diarrhea. The underused simple solution. N Engl J Med. 1990;323:891–4.
King CK, Glass R, Bresee JS. Managing acute gastroenteritis among children: oral rehydration, maintenance, and nutritional therapy. MMWR Recomm Rep. 2003;52:1–16.
Acknowledgments
We express our gratitude to all the people at the institutions who participated in the study.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Itou, K., Fukuyama, T., Sasabuchi, Y. et al. Safety and efficacy of oral rehydration therapy until 2 h before surgery: a multicenter randomized controlled trial. J Anesth 26, 20–27 (2012). https://doi.org/10.1007/s00540-011-1261-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00540-011-1261-x