Abstract
Background
Tertiary lymphoid structure (TLS) reflects an intense immune response against cancer, which correlates with favorable patient survival. However, the association of TLS with tumor-infiltrating lymphocytes (TILs) and clinical outcomes has not been investigated comprehensively in pancreatic ductal adenocarcinoma (PDAC).
Methods
We utilized an integrative molecular pathological epidemiology database on 162 cases with resected PDAC, and examined TLS in relation to levels of TILs, patient survival, and treatment response. In whole-section slides, we assessed the formation of TLS and conducted immunohistochemistry for tumor-infiltrating T cells (CD4, CD8, CD45RO, and FOXP3). As confounding factors, we assessed alterations of four main driver genes (KRAS, TP53, CDKN2A [p16], and SMAD4) using next-generation sequencing and immunohistochemistry, and tumor CD274 (PD-L1) expression assessed by immunohistochemistry.
Results
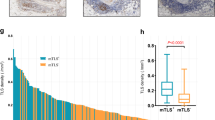
TLSs were found in 112 patients with PDAC (69.1%). TLS was associated with high levels of CD4+ TILs (multivariable odds ratio [OR], 3.50; 95% confidence interval [CI] 1.65–7.80; P = 0.0002), CD8+ TILs (multivariable OR, 11.0; 95% CI 4.57–29.7, P < 0.0001) and CD45RO+ TILs (multivariable OR, 2.65; 95% CI 1.25–5.80, P = 0.01), but not with levels of FOXP3+ TILs. TLS was associated with longer pancreatic cancer-specific survival (multivariable hazard ratio, 0.37; 95% CI 0.25–0.56, P < 0.0001) and favorable outcomes of adjuvant S-1-treatment. TLS was not associated with driver gene alterations but tumor CD274 negative expression.
Conclusions
Our comprehensive data supports the surrogacy of TLS for vigorous anti-tumor immune response characterized by high levels of helper and cytotoxic T cells and their prognostic role.


Similar content being viewed by others
Data availability
The sequence data reported are available in the DNA Data Bank of Japan (DDBJ) Sequenced Read Archive under the accession number DRA011317.
References
Mizrahi JD, Surana R, Valle JW, et al. Pancreatic cancer. Lancet. 2020;395:2008–20.
Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423–37.
Ziani L, Chouaib S, Thiery J. Alteration of the antitumor immune response by cancer-associated fibroblasts. Front Immunol. 2018;9:414.
Foucher ED, Ghigo C, Chouaib S, et al. Pancreatic ductal adenocarcinoma: A strong imbalance of Good and bad immunological cops in the tumor microenvironment. Front Immunol. 2018;9:1044.
Ino Y, Yamazaki-Itoh R, Shimada K, et al. Immune cell infiltration as an indicator of the immune microenvironment of pancreatic cancer. Br J Cancer. 2013;108:914–23.
Yang JJ, Hu ZG, Shi WX, et al. Prognostic significance of neutrophil to lymphocyte ratio in pancreatic cancer: a meta-analysis. World J Gastroenterol. 2015;21:2807–15.
Colbeck EJ, Ager A, Gallimore A, et al. Tertiary lymphoid structures in cancer: drivers of antitumor immunity, immunosuppression, or bystander sentinels in disease? Front Immunol. 2017;8:1830.
Dieu-Nosjean MC, Giraldo NA, Kaplon H, et al. Tertiary lymphoid structures, drivers of the anti-tumor responses in human cancers. Immunol Rev. 2016;271:260–75.
Ager A. High endothelial venules and other blood vessels: critical regulators of lymphoid organ development and function. Front Immunol. 2017;8:45.
Martinet L, Garrido I, Filleron T, et al. Human solid tumors contain high endothelial venules: association with T− and B- lymphocyte infiltration and favorable prognosis in breast cancer. Cancer Res. 2011;71:5678–87.
Lee HJ, Kim JY, Park IA, et al. Prognostic significance of tumor-infiltrating lymphocytes and the tertiary lymphoid structures in HER2-positive breast cancer treated with adjuvant trastuzumab. Am J Clin Pathol. 2015;144:278–88.
Germain C, Gnjatic S, Tamzalit F, et al. Presence of B cells in tertiary lymphoid structures is associated with a protective immunity in patients with lung cancer. Am J Respir Crit Care Med. 2014;189:832–44.
Goc J, Germain C, Vo-Bourgais TKD, et al. Dendritic cells in tumor- associated tertiary lymphoid structures signal a Th1 cytotoxic immune contexture and license the positive prognostic value of infiltrating CD8+T cells. Cancer Res. 2014;74:705–15.
Di Caro GD, Bergomas F, Grizzi F, et al. Occurrence of tertiary lymphoid tissue is associated with T-cell infiltration and predicts better prognosis in early-stage colorectal cancers. Clin Cancer Res. 2014;20:2147–58.
Posch F, Silina K, Leibl S, et al. Maturation of tertiary lymphoid structures and recurrence of stage II and III colorectal cancer. Oncoimmunology. 2018;7: e1378844.
Hiraoka N, Ino Y, Yamazaki-Itoh R, et al. Intratumoral tertiary lymphoid organ is a favourable prognosticator in patients with pancreatic cancer. Br J Cancer. 2015;112:1782–90.
Kuwabara S, Tsuchikawa T, Nakamura T, et al. Prognostic relevance of tertiary lymphoid organs following neoadjuvant chemoradiotherapy in pancreatic ductal adenocarcinoma. Cancer Sci. 2019;110:1853–62.
Waddell N, Pajic M, Patch AM, et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature. 2015;518:495–501.
Qian ZR, Rubinson DA, Nowak JA, et al. Association of alterations in main driver genes with outcomes of patients with resected pancreatic ductal adenocarcinoma. JAMA Oncol. 2018;4: e173420.
Chen H, Chong W, Teng C, et al. The immune response-related mutational signatures and driver genes in non-small-cell lung cancer. Cancer Sci. 2019;110:2348–56.
Luen S, Virassamy B, Savas P, et al. The genomic landscape of breast cancer and its interaction with host immunity. Breast. 2016;29:241–50.
Giannakis M, Mu XJ, Shukla SA, et al. Genomic correlates of immune-cell infiltrates in colorectal carcinoma. Cell Rep. 2016;15:857–65.
Jiang X, Wang J, Deng X, et al. Role of the tumor microenvironment in PD-L1/PD-1-mediated tumor immune escape. Mol Cancer. 2019;18:10.
Iwatate Y, Hoshino I, Yokota H, et al. Radiogenomics for predicting p53 status, PD-L1 expression, and prognosis with machine learning in pancreatic cancer. Br J Cancer. 2020;123:1253–61.
Petrackova A, Vasinek M, Sedlarikova L, et al. Standardization of sequencing coverage depth in NGS: recommendation for detection of clonal and subclonal mutations in Cancer Diagnostics. Front Oncol. 2019;9:851.
Cancer Genome Atlas Research Network. Electronic address: andrew_aguirre@dfci.harvard.edu, Cancer Genome Atlas Research Network. Integrated genomic characterization of pancreatic ductal adenocarcinoma. Cancer Cell. 2017;32:185–203.e13.
Yang SR, Lin CY, Stehr H, et al. Comprehensive genomic profiling of malignant effusions in patients with metastatic lung adenocarcinoma. J Mol Diagn. 2018;20:184–94.
Suzuki H, Aoki K, Chiba K, et al. Mutational landscape and clonal architecture in grade II and III gliomas. Nat Genet. 2015;47:458–68.
Mazaika E, Homsy J. Digital droplet PCR: CNV analysis and other applications. Curr Protoc Hum Genet. 2014;82:7.24.1-13.
Lassaletta A, Zapotocky M, Mistry M, et al. Therapeutic and prognostic implications of BRAF V600E in pediatric low-grade gliomas. J Clin Oncol. 2017;35:2934–41.
Tsujimae M, Masuda A, Ikegawa T, et al. Comprehensive analysis of molecular biologic characteristics of pancreatic ductal adenocarcinoma concomitant with intraductal papillary mucinous neoplasm. Ann Surg Oncol. 2022;29:4924–34.
Yachida S, White CM, Naito Y, et al. Clinical significance of the genetic landscape of pancreatic cancer and implications for identification of potential long-term survivors. Clin Cancer Res. 2012;18:6339–47.
Ohtsubo K, Watanabe H, Yamaguchi Y, et al. Abnormalities of tumor suppressor gene p16 in pancreatic carcinoma: immunohistochemical and genetic findings compared with clinicopathological parameters. J Gastroenterol. 2003;38:663–71.
Tascilar M, Skinner HG, Rosty C, et al. The SMAD4 protein and prognosis of pancreatic ductal adenocarcinoma. Clin Cancer Res. 2001;7:4115–21.
Ying S, Humbert M, Barkans J, et al. Expression of IL-4 and IL-5 mRNA and protein product by CD4+ and CD8+ T cells, eosinophils, and mast cells in bronchial biopsies obtained from atopic and nonatopic (intrinsic) asthmatics. J Immunol. 1997;158:3539–44.
Vonderheide RH. The Immune Revolution: A Case for Priming. Not Checkpoint Cancer Cell. 2018;33:563–9.
Zitvogel L, Galluzzi L, Kepp O, et al. Type I interferons in anticancer immunity. Nat Rev Immunol. 2015;15:405–14.
Hotta K, Sho M, Fujimoto K, et al. Prognostic significance of CD45RO+ memory T cells in renal cell carcinoma. Br J Cancer. 2011;105:1191–6.
Tan AH, Goh SY, Wong SC, et al. T helper cell-specific regulation of inducible costimulator expression via distinct mechanisms mediated by T-bet and GATA-3. J Biol Chem. 2008;283:128–36.
Masugi Y, Abe T, Ueno A, et al. Characterization of spatial distribution of tumor-infiltrating CD8 + T cells refines their prognostic utility for pancreatic cancer survival. Mod Pathol. 2019;32:1495–507.
Takada K, Kashiwagi S, Asano Y, et al. Clinical verification of the relationship between smoking and the immune microenvironment of breast cancer. J Transl Med. 2019;17:13.
Liu C, Xu B, Li Q, et al. Smoking history influences the prognostic value of peripheral naïve CD4+ T cells in advanced non-small cell lung cancer. Cancer Cell Int. 2019;19:176.
Pineda S, Maturana EL, Yu K, et al. Tumor-Infiltrating B- and T-Cell Repertoire in Pancreatic Cancer Associated With Host and Tumor Features. Front Immunol. 2021;12: 730746.
Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017;541:321–30.
Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7–H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800.
Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39:1–10.
Mundry CS, Eberle KC, Singh PK, et al. Local and systemic immunosuppression in pancreatic cancer: targeting the stalwarts in tumor’s arsenal. Biochim Biophys Acta Rev Cancer. 2020;1874: 188387.
Sautès-Fridman C, Petitprez F, Calderaro J, et al. Tertiary lymphoid structures in the era of cancer immunotherapy. Nat Rev Cancer. 2019;19:307–25.
Farren MR, Mace TA, Geyer S, et al. Systemic immune activity predicts overall survival in treatment-naïve patients with metastatic pancreatic cancer. Clin Cancer Res. 2016;22:2565–74.
Lee KH, Kim EY, Yun JS, et al. The prognostic and predictive value of tumor-infiltrating lymphocytes and hematologic parameters in patients with breast cancer. BMC Cancer. 2018;18:938.
Huang Y, Lou XY, Zhu YX, et al. Local environment in biopsy better predict the pathological response to neoadjuvant chemoradiotherapy in rectal cancer. Biosci Rep 2019; 39.
Uesaka K, Boku N, Fukutomi A, et al. Adjuvant chemotherapy of S-1 versus gemcitabine for resected pancreatic cancer: a phase 3, open-label, randomised, non-inferiority trial (JASPAC 01). Lancet. 2016;388:248–57.
Zitvogel L, Apetoh L, Ghiringhelli F, et al. Immunological aspects of cancer chemotherapy. Nat Rev Immunol. 2008;8:59–73.
Adachi Y, Taketani S, Oyaizu H, et al. Apoptosis of colorectal adenocarcinoma induced by 5-FU and/or IFN-gamma through caspase 3 and caspase 8. Int J Oncol. 1999;15:1191–6.
Dunn GP, Koebel CM, Schreiber RD. Interferons, immunity and cancer Immunoediting. Nat Rev Immunol. 2006;6:836–48.
Kursunel MA, Esendagli G. The untold story of IFN-γ in cancer biology. Cytokine Growth Factor Rev. 2016;31:73–81.
Choudhry H, Helmi N, Abdulaal WH, et al. Prospects of IL-2 in cancer immunotherapy. Biomed Res Int. 2018; p. 9056173.
Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015;348:69–74.
Pu N, Chen Q, Gao S, Liu G, Zhu Y, Yin L, et al. Genetic landscape of prognostic value in pancreatic ductal adenocarcinoma microenvironment. Ann Transl Med. 2019;7:645.
Wartenberg M, Cibin S, Zlobec I, et al. Integrated genomic and immunophenotypic classification of pancreatic cancer reveals three distinct subtypes with prognostic/predictive significance. Clin Cancer Res. 2018;24:4444–54.
Cheng H, Fan K, Luo G, et al. Kras G12D mutation contributes to regulatory T cell conversion through activation of the MEK/ERK pathway in pancreatic cancer. Cancer Lett. 2019;446:103–11.
Acknowledgements
The authors would like to thank Takashi Omori (Clinical & Translational Research Center, Kobe University), Nobuyuki Kakiuchi (Department of Pathology and Tumor Biology, Kyoto University) and Yohei Masugi (Department of Pathology, Keio University School of Medicine) for their valuable comments. We would like to thank Editage (www.edita ge.jp) for the English language editing.
Funding
This work was supported by JSPS KAKENHI (Grants-in-Aid for Scientific Research), Grant no. 19K08444 (A.M.), and Grant no. 19H03698 (Y.K.). This work was also supported by Pancreas Research Foundation of Japan (A.M.).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by TT, JI, TI, ST, RN, YY, SA, MT, KY, SA, MG, SM, NI, HU, SK and KN. The first draft of the manuscript was written by TT, and AM designed the study concept, analyzed data, and wrote the manuscript. MK and TI reviewed all slides in the study and advised on pathological diagnosis as needed. TH, HT, KS, HS, AS, TK, YU, TF and YK were involved in study supervision and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no potential conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
535_2022_1939_MOESM1_ESM.tif
Supplementary Fig. 1 Representative immuno-histochemistry images. a Typical images of high or low levels of CD4+, CD8+, CD45RO+ and FOXP3+ TILs. b Typical images of presence or absence of CD274 (PD-L1) expression. c Typical images of intact or altered (lost or over expression) of protein expression of TP53, CDKN2A and SMAD4. Original magnification (a-c, 200×). Scale bars, 50 μm (a-c). PDL1, Programmed death ligand 1; TILs, tumor-infiltrating lymphocytes
535_2022_1939_MOESM2_ESM.tif
Supplementary Fig. 2 CSS of PDAC patients according to the intratumoral TLS and peritumoral TLS. Intratumoral TLSs were detected within and around the tumor; peritumoral TLSs were detected only around the tumor. The CSS curves were estimated using the Kaplan–Meier method, and the significant differences between the two groups were evaluated using a log-rank test. The number of patients at risk is shown in the CSS curves. CSS, cancer-specific survival; PDAC, pancreatic ductal adenocarcinoma; TLS, tertiary lymphoid structure
535_2022_1939_MOESM3_ESM.tif
Supplementary Fig. 3 CSS of PDAC patients according to the level of NLR or extent of CD8+ TILs. The CSS of PDAC patients according to high or low NLR were investigated. The differences of the CSS in adjuvant chemotherapy among the groups treated with S-1, gemcitabine, and non-treated groups according to the high or low NLR were also examined. a All patients b Patients with low NLR c Patients with high NLR. The CSS of PDAC according to the high or low levels of CD8+ TILs were investigated. The differences of the CSS in adjuvant chemotherapy among the groups treated with S-1, gemcitabine, and non-treated groups according to the high or low levels of CD8+ TILs were also examined. d All patients e Patients with high levels of CD8+ TILs f Patients with low levels of CD8+ TILs. The CSS curves were estimated using the Kaplan-Meier method, and the significant differences between the two groups were evaluated by a log-rank test. The number of patients at risk is shown in the CSS curves. CSS, cancer-specific survival; NLR, neutrophil-to lymphocyte ratio; PDAC, pancreatic ductal adenocarcinoma; TILs, tumor-infiltrating lymphocytes
535_2022_1939_MOESM4_ESM.tif
Supplementary Fig. 4 In situ hybridization for IFN-gamma and IL-2, and apoptosis assay. a Typical images of IFN-gamma and IL-2 expression in TLS- present and absent patients. IFN-gamma-positive cells and IL-2-positive cells show distinct red staining. b The expression levels of IFN-gamma or IL-2 per cm2 in TLS-present patients (n=5) and TLS-absent patients (n=5) were counted. Mean ± SD (bars). *, P < 0.05. c Double immunohistochemistry-in situ hybridization. To distinguish between IHC of CD4+ and CD8+ T cells (brown) and ISH of IFN-gamma (pink) more clearly, brown is changed to green using color replacement tools of Photoshop. IFN-gamma mRNA (pink arrowhead) was localized to CD4+ and CD8+ T cells (green). d TUNEL assays were performed 7 days after the addition of IFN-gamma (100 units/mL), IL-2 (500 units/ml), 5-FU (0.05 mg/mL), and their combination to detect the apoptotic cells. TUNEL analysis showed a significant difference in cell numbers at day 7 between the combination therapy group and other treatment groups. Mean ± SD (bars). **, P < 0.001, ***, P < 0.01. Original magnification (a, c, 400×). Scale bars, 20 μm. IFN, interferon; IL, interleukin; PDAC, pancreatic ductal adenocarcinoma; SD, standard deviation; TLS, tertiary lymphoid structure
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tanaka, T., Masuda, A., Inoue, J. et al. Integrated analysis of tertiary lymphoid structures in relation to tumor-infiltrating lymphocytes and patient survival in pancreatic ductal adenocarcinoma. J Gastroenterol 58, 277–291 (2023). https://doi.org/10.1007/s00535-022-01939-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-022-01939-8