Abstract
Purpose
Paclitaxel is associated with an acute pain syndrome (P-APS- and chronic chemotherapy-induced peripheral neuropathy (CIPN). P-APS is associated with higher risk of CIPN. Omega-3 fatty acids have well-established anti-inflammatory and neuroprotective properties. The primary purpose of this pilot study was to assess whether omega-3 fatty acids could decrease P-APS and thus CIPN.
Methods
Patients scheduled to receive weekly paclitaxel for breast cancer were randomized to receive 4 g of omega-3 acid ethyl esters (Lovaza) or placebo, beginning 1 week prior and continued until paclitaxel was stopped. Patients completed acute pain questionnaires at baseline, daily after each treatment, and 1 month after completion of therapy.
Results
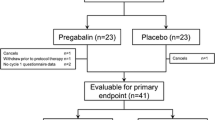
Sixty patients (49 evaluable) were randomized to treatment versus placebo. Seventeen (68.0%) patients receiving the omega-3 fatty acids intervention experienced P-APS, compared to 15 (62.5%) of those receiving placebo during the first week of treatment (p = 0.77). Over the full 12-week study, 21 (84.0%) patients receiving the omega-3 fatty acid intervention experienced P-APS, compared to 21 (87.5%) of those receiving placebo (p = 1.0). Secondary outcomes suggested that those in the intervention arm used more over-the-counter analgesics (OR: 1.65, 95% CI: 0.72–3.78, p = 0.23), used more opiates (OR: 2.06, 95% CI: 0.55–7.75, p = 0.28), and experienced higher levels of CIPN (12.8, 95% CI: 7.6–19.4 vs. 8.4, 95% CI: 4.6–13.2, p = 0.21).
Conclusions
The results of this pilot study do not support further study of the use of omega-3 fatty acids for the prevention of the P-APS and CIPN.
Trial registration
Number: NCT01821833



Similar content being viewed by others
References
Garrison JA et al (2003) Myalgias and arthralgias associated with paclitaxel. Oncology (Williston Park) 17(2):271–277; discussion 281-282, 286–288
Loprinzi CL et al (2011) Natural history of paclitaxel-associated acute pain syndrome: prospective cohort study NCCTG N08C1. JCO 29(11):1472–1478. https://doi.org/10.1200/JCO.2010.33.0308
Calder PC (2008) Polyunsaturated fatty acids, inflammatory processes and inflammatory bowel diseases. Mol Nutr Food Res 52(8):885–897. https://doi.org/10.1002/mnfr.200700289
Reeves BN et al (2012) Further data supporting that paclitaxel-associated acute pain syndrome is associated with development of peripheral neuropathy. Cancer 118(20):5171–5178. https://doi.org/10.1002/cncr.27489
Chiu N et al (2018) A prospective study of docetaxel-associated pain syndrome. Support Care Cancer 26(1):203–211. https://doi.org/10.1007/s00520-017-3836-z
Bindu S, Mazumder S, Bandyopadhyay U (2020) Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: a current perspective. Biochem Pharmacol 180:114147. https://doi.org/10.1016/j.bcp.2020.114147
Markman M, Kennedy A, Webster K, Kulp B, Peterson G, Belinson J (1999) Use of low-dose oral prednisone to prevent paclitaxel-induced arthralgias and myalgias. Gynecol Oncol 72(1):100–101. https://doi.org/10.1006/gyno.1998.5226
Jacobson SD et al (2003) Glutamine does not prevent paclitaxel-associated myalgias and arthralgias. J Support Oncol 1(4):274–278
Martoni A, Zamagni C, Gheka A, Pannuti F (1993) Antihistamines in the treatment of taxol-induced paroxystic pain syndrome. JNCI 85(8):676–677. https://doi.org/10.1093/jnci/85.8.676
Samuels N, Ben-Arye E (2020) Integrative approaches to chemotherapy-induced peripheral neuropathy. Curr Oncol Rep 22(3):23. https://doi.org/10.1007/s11912-020-0891-2
Yoshida T, Sawa T, Ishiguro T, Horiba A, Minatoguchi S, Fujiwara H (2009) The Efficacy of prophylactic Shakuyaku-Kanzo-to for myalgia and arthralgia following carboplatin and paclitaxel combination chemotherapy for non-small cell lung cancer. Support Care Cancer 17(3):315–320. https://doi.org/10.1007/s00520-008-0508-z
Brami C, Bao T, Deng G (2016) Natural products and complementary therapies for chemotherapy-induced peripheral neuropathy: a systematic review. Crit Rev Oncol Hematol 98:325–334. https://doi.org/10.1016/j.critrevonc.2015.11.014
Schloss J, Colosimo M, Vitetta L (2016) New insights into potential prevention and management options for chemotherapy-induced peripheral neuropathy. Asia Pac J Oncol Nurs 3(1):73–85. https://doi.org/10.4103/2347-5625.170977
Cavaletti G, Cavalletti E, Montaguti P, Oggioni N, De Negri O, Tredici G (1997) Effect on the peripheral nervous system of the short-term intravenous administration of paclitaxel in the rat. Neurotoxicology 18(1):137–145
Peters CM et al (2007) Intravenous paclitaxel administration in the rat induces a peripheral sensory neuropathy characterized by macrophage infiltration and injury to sensory neurons and their supporting cells. Exp Neurol 203(1):42–54. https://doi.org/10.1016/j.expneurol.2006.07.022
Goldberg RJ, Katz J (2007) A meta-analysis of the analgesic effects of omega-3 polyunsaturated fatty acid supplementation for inflammatory joint pain. Pain 129(1–2):210–223. https://doi.org/10.1016/j.pain.2007.01.020
Kwiatkowska B, Maślińska M (2020) The place of omega-3 and omega-6 acids in supplementary treatment of inflammatory joint diseases. Reumatologia 58(1):34–41. https://doi.org/10.5114/reum.2020.93511
Silva RV et al (2017) Long-chain omega-3 fatty acids supplementation accelerates nerve regeneration and prevents neuropathic pain behavior in mice. Front Pharmacol 8:723. https://doi.org/10.3389/fphar.2017.00723
Hong S, Gronert K, Devchand PR, Moussignac R-L, Serhan CN (2003) Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood, and glial cells. J Biol Chem 278(17):14677–14687. https://doi.org/10.1074/jbc.M300218200
Novel docosanoids inhibit brain ischemia-reperfusion-mediated leukocyte infiltration and pro-inflammatory gene expression - ScienceDirect. https://www.sciencedirect.com/science/article/pii/S0021925820826696?via%3Dihub. Accessed 20 Dec. 2021
Ji R-R, Xu Z-Z, Strichartz G, Serhan CN (2011) Emerging roles of resolvins in the resolution of inflammation and pain. Trends Neurosci 34(11):599–609. https://doi.org/10.1016/j.tins.2011.08.005
Bradberry JC, Hilleman DE (2013) Overview of omega-3 fatty acid therapies. P T 38(11):681–691
Dyall SC, Michael-Titus AT (2008) Neurological benefits of omega-3 fatty acids. NeuroMolecular Med 10(4):219–235. https://doi.org/10.1007/s12017-008-8036-z
Bazan NG, Molina MF, Gordon WC (2011) Docosahexaenoic acid signalolipidomics in nutrition: significance in aging, neuroinflammation, macular degeneration, Alzheimer’s, and other neurodegenerative diseases. Annu Rev Nutr 31:321–351. https://doi.org/10.1146/annurev.nutr.012809.104635
King VR, Huang WL, Dyall SC, Curran OE, Priestley JV, Michael-Titus AT (2006) Omega-3 fatty acids improve recovery, whereas omega-6 fatty acids worsen outcome, after spinal cord injury in the adult rat. J Neurosci 26(17):4672–4680. https://doi.org/10.1523/JNEUROSCI.5539-05.2006
Lim S-N, Huang W, Hall JCE, Ward RE, Priestley JV, Michael-Titus AT (2010) The acute administration of eicosapentaenoic acid is neuroprotective after spinal cord compression injury in rats. Prostaglandins Leukot Essent Fat Acids 83(4–6):193–201. https://doi.org/10.1016/j.plefa.2010.08.003
Gladman SJ et al (2012) Improved outcome after peripheral nerve injury in mice with increased levels of endogenous ω-3 polyunsaturated fatty acids. J Neurosci 32(2):563–571. https://doi.org/10.1523/JNEUROSCI.3371-11.2012
Kyle DJ, Schaefer E, Patton G, Beiser A (1999) Low serum docosahexaenoic acid is a significant risk factor for Alzheimer’s dementia. Lipids 34 Suppl:S245. https://doi.org/10.1007/BF02562306
Barberger-Gateau P, Letenneur L, Deschamps V, Pérès K, Dartigues J-F, Renaud S (2002) Fish, meat, and risk of dementia: cohort study. BMJ 325(7370):932–933
Chemotherapy-induced peripheral neuropathy - EORTC - Quality of Life: EORTC – Quality of Life. https://qol.eortc.org/questionnaire/qlq-cipn20/, https://qol.eortc.org/questionnaire/qlq-cipn20/. Accessed 18 Jun. 2023
Quasthoff S, Hartung HP (2002) Chemotherapy-induced peripheral neuropathy. J Neurol 249(1):9–17. https://doi.org/10.1007/PL00007853
Altman DG (1990) Practical Statistics for Medical Research. CRC Press
Ibrahim EY, Ehrlich BE (2020) Prevention of chemotherapy-induced peripheral neuropathy: a review of recent findings. Crit Rev Oncol Hematol 145:102831. https://doi.org/10.1016/j.critrevonc.2019.102831
Staff NP, Grisold A, Grisold W, Windebank AJ (2017) Chemotherapy-induced peripheral neuropathy: a current review. Ann Neurol 81(6):772–781. https://doi.org/10.1002/ana.24951
Ghoreishi Z et al (2012) Omega-3 fatty acids are protective against paclitaxel-induced peripheral neuropathy: a randomized double-blind placebo controlled trial. BMC Cancer 12(1):355. https://doi.org/10.1186/1471-2407-12-355
Anoushirvani AA, Poorsaadat L, Aghabozorgi R, Kasravi M (2018) Comparison of the effects of omega 3 and vitamin E on palcitaxel-induced peripheral neuropathy. Open Access Maced J Med Sci 6(10):1857–1861. https://doi.org/10.3889/oamjms.2018.333
Mercieca-Bebber R, King MT, Calvert MJ, Stockler MR, Friedlander M (2018) The importance of patient-reported outcomes in clinical trials and strategies for future optimization. Patient Relat Outcome Meas 9:353–367. https://doi.org/10.2147/PROM.S156279
Loprinzi CL et al (2020) Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: ASCO guideline update. JCO 38(28):3325–3348. https://doi.org/10.1200/JCO.20.01399
Funding
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under the Award Number UG1CA189823 (Alliance for Clinical Trials in Oncology NCORP Grant), UG1CA232760, and partially supported by the UNM Comprehensive Cancer Center Support Grant NCI P30CA118100, and the Biostatistics shared resource. https://acknowledgments.alliancefound.org.
Author information
Authors and Affiliations
Contributions
Z.D., J.L., L.C., and D.B. conceived the initial trial design. V.P. performed data analysis and prepared figures and tables. B.T. wrote the manuscript with significant edits from U.B. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was performed in line with the principles of the Declaration of Helsinki. This study was approved by the University of New Mexico Health Sciences Center Institutional Review Board [UNM HRPO] (#19-562) and monitored by the Data Safety Monitoring Board.
Informed consent
Was obtained from all individual participants included in the study.
Competing interests
The authors declare no competing interests.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tawfik, B., Dayao, Z.R., Brown-Glaberman, U.A. et al. A pilot randomized, placebo-controlled, double-blind study of omega-3 fatty acids to prevent paclitaxel-associated acute pain syndrome in breast cancer patients: Alliance A22_Pilot2. Support Care Cancer 31, 637 (2023). https://doi.org/10.1007/s00520-023-08082-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00520-023-08082-x