Abstract
Purpose
The purpose of this scoping review is to appraise the published literature on taste disturbance in patients with advanced cancer, with the specific objectives being to determine its prevalence, clinical features and complications.
Methods
This scoping review was conducted using the recommended methodological framework. A detailed search of databases (Medline, Embase, CINAHL and PsycInfo) was conducted to identify eligible studies: eligible studies needed to include patients with advanced cancer and needed to include details of clinical features and/or complications of taste disturbance. Standard bibliographic/systematic review software was used to store the records and manage the review process, respectively.
Results
Twenty-five studies were identified from the database searches. The studies identified included eight physical and/or psychological symptom studies, six symptom cluster studies, five oral symptom studies and six taste and/or smell specific studies. Detailed data is presented on the clinical features and complications of taste disturbance and on the symptom clusters involving taste disturbance in this cohort of patients.
Conclusion
This scoping review identified a relatively small number of relevant studies involving a relatively small number of participants. Nevertheless, it confirms that taste disturbance is a common problem in patients with advanced cancer and is associated with significant morbidity because of the primary condition and the associated complications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Taste is defined as “the perception derived when chemical molecules stimulate taste receptors in areas of the tongue, soft palate and oropharyngeal region of the oral cavity to perceive the five basic taste qualities: “sweet, sour, salty, bitter and umami” [1]. The terms “taste” and “flavour” are often used interchangeably in real life, clinical practice [2, 3] and the medical literature [4]. However, taste and flavour are distinct entities with flavour encompassing a combination of taste, smell, texture and temperature [5]. Food hedonics encompasses the palatability of foods, where ‘liking’ food is defined as “the immediate experience or anticipation of pleasure from the orosensory stimulation of eating a food” [6]. Taste disturbance can present as a distortion of normal taste sensation (dysgeusia), a reduction in taste sensation (hypogeusia), increased taste sensation (hypergeusia) or as an absence of taste sensation (ageusia) [7].
Taste disturbance can be evaluated subjectively through patient-reported assessments or by objective assessments. In the clinical setting, it can be measured objectively by measuring oral taste sensitivity to tastants through thresholds to some or all the five basic tastes [8]. No independently diagnostic biochemical measures are available [9].
Taste disturbance is relatively common in the general population; data from the US National Health and Nutrition Examination Survey (2013–2014) suggested that the prevalence of objective taste disturbance defined as failing to identify quinine/bitter taste, was 17.3% amongst the US general population [10]. There are a number of different causes for taste disturbance [11], including dental (periodontal) disease, upper respiratory tract infection, medication-related (e.g. antihypertensives, antimicrobials), dental procedure-related trauma, tonsillectomy, middle ear surgery, Bell’s palsy and burning mouth syndrome. Taste and smell disorders often occur simultaneously [9].
Equally, taste disturbance is especially common in patients with cancer [12]. Taste disturbance may occur at diagnosis (treatment naive patients) [13], during anticancer treatment [14], following anticancer treatment (chronic side effect) [15], at disease progression and into cancer survivorship [16]. There are a number of potential causes of taste disturbance in cancer patients, including direct effects of the cancer, indirect effects of the cancer (i.e. paraneoplastic syndromes), adverse effects of anticancer treatments, adverse effects of supportive care measures and co-morbidities (and their management) [17]. It should be noted that the literature already contains a number of reviews relating to taste disturbance in specific subgroups of cancer patients (e.g. lung cancer) [18,19,20] and specific anticancer treatments (e.g. head and neck radiotherapy) [21].
It has been known for some time that patients with advanced cancer often develop taste disturbances [22]. However, taste disturbance is considered an “orphan symptom”, which is defined as “symptoms not regularly assessed in clinical practice, and consequently little studied and not properly treated” [23]. The aim of this scoping review is to appraise the published literature on taste disturbance in patients with advanced cancer, with the specific objectives being to determine its prevalence, clinical features (i.e. subjective and objective) and impact on the patients (i.e. physical and psycho-social). It appears that there is no analogous scoping review within the clinical literature.
Methods
This scoping review was conducted using the methodological framework developed by Arksey and O’Malley [24] and incorporating updated guidance on this methodology [25]. The PRISMA Extension for Scoping Reviews (PRISMA-ScR) was used to report the findings [26].
Search strategy
A detailed search of four electronic databases (Medline, Embase, CINAHL and PsycInfo) was conducted in October 2022. The search strategy for Medline is shown in Appendix. The search strategy was adapted as needed for each electronic database. The search was re-run in January 2023 to check for any new references.
Study eligibility criteria
Eligible studies needed to include patients with advanced cancer, as defined by the National Cancer Institute/NCI, USA: “Cancer that is unlikely to be cured or controlled with treatment. The cancer may have spread from where it first started to nearby tissue, lymph nodes, or distant parts of the body” [27]. Studies which included mixed groups of patients with cancer were excluded, unless results for the patients with advanced cancer were separately reported. Studies which focussed on patients with advanced head and neck cancer, and studies that focussed on cancer patients receiving anticancer treatment were excluded. Eligible studies also needed to include details of clinical features and/or complications of taste disturbance. Studies reporting mixed chemosensory changes were excluded, unless results for taste changes were separately reported. Case reports, review articles and other records without original information were not included. Studies involving children (< 19 years) and non-English language studies were excluded.
Data management and synthesis
EndNote 20™ bibliographic software (Clarivate Analytics LLP, USA) was used to store the records retrieved from all the searches. Screening of the records was completed using Covidence systematic review software (Veritas Health Innovation, Australia).
Two reviewers (MH and AD) independently screened the titles and abstracts for full text articles to review. A third reviewer (MC) was available to resolve potential conflicts relating to record inclusion. Two reviewers (MH and AS) independently reviewed the full text articles and extracted the relevant information using a review-specific template. A third reviewer (AD) was available to resolve conflicts relating to data extraction.
The reference lists of all retrieved full text articles, pertinent chapters in major palliative care textbooks and pertinent sections of major palliative care guidelines were hand searched for other relevant records. Other sources of relevant records included the researchers themselves.
Results
Search results
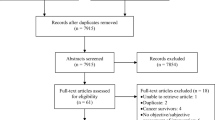
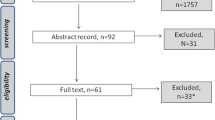
The search strategy identified 8042 references, although only 99 full text articles were retrieved (see Fig. 1). Seven studies were identified from the database searches and had their data extracted [28,29,30,31,32,33,34]. Another eighteen studies were identified from handsearching [35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52]. The studies identified included eight physical and/or psychological symptom studies [29, 35, 42, 46, 47, 49, 51, 52], six symptom cluster studies [28, 32, 37, 43, 48, 50], five oral symptom studies [33, 34, 36, 38, 39] and six taste and/or smell specific studies [30, 31, 40, 41, 44, 45] (Tables 1 and 2). Several “duplicate” records were identified amongst the retrieved full text articles; some were conference abstracts, some were journal articles reporting “early” results, and some were journal articles reporting different analyses/subsets of results.
Assessment
The six taste and/or smell specific studies were generally small (median: 48 participants, range: 15–192 participants). All studies involved a subjective assessment, whilst three studies included an objective assessment [31, 44, 45]. Currently, there is no validated tool to assess taste disturbances in this group of patients; four studies [30, 41, 44, 45] utilised the Taste and Smell Survey [53]; and the other studies used non-validated, study-specific questionnaires. Similarly, there is no agreed method for assessing objective taste disturbances in this group of patients (or indeed other groups of patients) [58]; two studies used commercial taste strips [44, 45] and one study used liquid tastants [31].
Importantly, the terminology used in the studies varied, which may have had an effect on the results obtained, especially prevalence statistics: “abnormal taste”, “absence of taste”, “altered taste”, “any taste disturbances”, “bad or altered taste”, “changes in my sense of taste”, “changes in food tasting”, “change in the way food tastes”, “a food tastes different than it used to”, “food tasted differently”, “persistent bad taste in my mouth”, “reduction of taste”, “taste alteration”, “taste change”, “taste disturbance(s)” and “taste problems”. No studies included questions regarding flavour, as opposed to taste. This issue has been highlighted in previous literature reviews involving other cohorts of oncology patients [59].
Epidemiology
The prevalence of taste disturbance varied widely in the studies included in this scoping review (median: 55%, range: 27–93%) [35, 44]. Alsirafy et al. reported that no patients reported this symptom on open questioning, although 27% patients gave a positive response on systematic assessment (with 41.5% of these patients reporting “moderate”/“severe” intensity) [35]. Importantly, certain studies reported that different assessment tools produced different prevalence figures [38, 44, 45].
Surprisingly, there was little data on factors affecting prevalence (e.g. demographics, cancer diagnosis, performance status and comorbidities). Walsh et al. reported no association between taste disturbance and age, sex or performance status in their patient database [60]. Interestingly, Tranmer et al. reported that taste disturbance was more common in patients with advanced cancer than patients with “end-stage” non-malignant disease [47]. There was limited data on the aetiology of taste disturbance in patients with advanced cancer, although one group of researchers reported an association between the severity of xerostomia (subjective sensation of dry mouth) and the severity of taste disturbance [61]. Furthermore, the same group of researchers reported an oral symptom cluster consisting of xerostomia and taste disturbance (see below) [38].
Symptom clusters
Table 2 shows studies reporting physical and/or psychological symptom clusters involving taste disturbance [28, 32, 37, 43, 48, 50]. The symptom clusters identified varied from study to study and varied within study (depending on the outcome measure chosen and the statistical method utilised) [28]. It should be noted that there are many other studies reporting physical and/or psychological symptom clusters in patients with advanced cancer, but which did not include the symptom of taste disturbance [62]. Davies et al. (2021) investigated symptom clusters involving specifically oral symptoms and found an association between the presence of taste disturbance and the presence of a “dirty mouth”, and “coating of tongue” (Spearman’s rank correlation coefficient = 0.7) and also an association between the frequency of taste disturbance and the frequency of “dry mouth” (Spearman’s rank correlation coefficient = 0.6) [38]. No analogous studies were identified in the literature.
Clinical features
Table 1 shows studies reporting the clinical features of taste disturbance [29,30,31, 33,34,35,36, 38,39,40,41,42, 44,45,46,47, 49, 51, 52]. It demonstrates that taste disturbance is usually a persistent symptom [38, 39, 44, 47, 49], is often moderate-to-severe in intensity [33,34,35, 38,39,40, 46, 47, 49] and is often associated with significant distress [38, 39, 42, 46, 47, 51]. It should be noted that there were many other studies reporting taste disturbance in patients with advanced cancer, but which did not include details about clinical features and/or complications.
Patients reported a variety of subjective taste disturbance, including ageusia [40, 44], hypogeusia [29, 31, 40, 41, 44, 45], hypergeusia [31, 41, 44, 45] and dysgeusia [29,30,31, 33,34,35,36, 38,39,40,41,42, 44,45,46,47, 49, 51, 52]. Furthermore, patients often reported more than one type of subjective taste disturbance, and these taste disturbances could affect some or all taste qualities (i.e. bitter, salt, sour and sweet) [31, 40, 41, 44, 45]. Taste disturbance often coexisted with a disturbance in the sense of smell [30, 41, 44, 45].
Impact of taste disturbances
Taste disturbance may have a major impact on the experience and pleasure associated with eating and drinking, with patients reporting aversions to a variety of foods (e.g. meat) and/or drinks (e.g. alcohol) [31, 63]. Some adopt compensatory strategies such as seasoning food in an attempt to make it palatable [64] or changing their behaviour by ‘taking control’ of when and what they ate [65].
Indeed, taste disturbance may have a major impact on nutritional intake [30, 41] and is one of the preeminent “nutritional impact symptoms” in patients with advanced cancer [38]. Moreover, taste disturbance appears to be a relevant issue in many patients with the cancer-related anorexia/cachexia syndrome [63], with the severity of taste disturbance greater in patients with refractory cachexia, than patients with pre-cachexia, cachexia or without cachexia [66].
Unsurprisingly, taste disturbance may be associated with low mood/depression [64], social isolation (i.e. avoidance of social eating) and an impaired quality of life [30, 41].
Discussion
This unique scoping review identified a relatively small number of relevant studies involving a relatively small number of participants. Nevertheless, it confirms that taste disturbance is a common problem in patients with advanced cancer and is associated with significant morbidity (because of the primary condition and the associated complications). The results suggest that this so-called “orphan” symptom warrants greater recognition from patients, family carers and especially healthcare professionals.
Importantly, taste disturbance may be amenable to treatment [67] which may result in increased enjoyment of eating and drinking, decreased morbidity and increased quality of life. In addition, resolution/improvement in taste disturbance may prevent/limit malnutrition, which is a major cause of death in this group of patients [68]. Thus, it is important to screen patients for taste disturbance and refer affected patients to an oncology dietician or other appropriate healthcare professional for further assessment/management.
This scoping review has also identified gaps in the current literature and topics for future research in this cohort of patients: (a) observational studies to determine the “risk factors” for taste disturbance (e.g. cancer diagnosis and performance status) — this data would facilitate targeted screening for the problem; (b) observational studies to determine the aetiologies of taste disturbance overall — this data would also facilitate targeted screening for the problem; (c) observational studies to determine the aetiologies of different subtypes of taste disturbance (e.g. ageusia and dysgeusia) — this data would facilitate targeted treatment for the problem; (d) observational studies of patient/family carers unmet needs and priorities for management; (e) development/validation studies of a taste-specific assessment tool for this group of patients — there is a need for a tool that not only assesses the problem but can also assess the response to treatment for the problem, utilising patient-reported outcome measures.
References
Breslin PA, Spector AC (2008) Mammalian taste perception. Curr Biol 18:R148–R155. https://doi.org/10.1016/j.cub.2007.12.017
Boltong A, Keast R, Aranda SK (2011) Talking about taste: how do oncology clinicians discuss and document taste problems? Cancer Forum 35:81–87
Galaniha LT, Nolden AA (2023) Taste loss in cancer patients: clinicians’ perceptions of educational materials and diagnostic tools. Support Care Cancer 31:349. https://doi.org/10.1007/s00520-023-07794-4
Boltong A, Keast RS, Aranda SK (2011) A matter of taste: making the distinction between taste and flavor is essential for improving management of dysgeusia. Support Care Cancer 19:441–442. https://doi.org/10.1007/s00520-011-1085-0
Boltong A, Keast R (2012) The influence of chemotherapy on taste perception and food hedonics: a systematic review. Cancer Treat Rev 38:152–163. https://doi.org/10.1016/j.ctrv.2011.04.008
Mela DJ (2006) Eating for pleasure or just wanting to eat? Reconsidering sensory hedonic responses as a driver of obesity. Appetite 47:10–17. https://doi.org/10.1016/j.appet.2006.02.006
Epstein JB, Barasch A (2010) Taste disorders in cancer patients: pathogenesis, and approach to assessment and management. Oral Oncol 46:77–81. https://doi.org/10.1016/j.oraloncology.2009.11.008
Hovan AJ, Williams PM, Stevenson-Moore P, Wahlin YB, Ohrn KE, Elting LS, Spijkervet FK, Brennan MT (2010) A systematic review of dysgeusia induced by cancer therapies. Support Care Cancer 18:1081–1087. https://doi.org/10.1007/s00520-010-09021
Henkin RI, Levy LM, Fordyce A (2013) Taste and smell function in chronic disease: a review of clinical and biochemical evaluations of taste and smell dysfunction in over 5000 patients at The Taste and Smell Clinic in Washington, DC. Am J Otolaryngol 34:477–489. https://doi.org/10.1016/j.amjoto.2013.04.006
Liu G, Zong G, Doty RL, Sun Q (2016) Prevalence and risk factors of taste and smell impairment in a nationwide representative sample of the US population: a cross-sectional study. BMJ Open 6:e013246. https://doi.org/10.1136/bmjopen-2016-013246
Barasch A, Epstein JB (2022) Evaluation of taste disorders. BMJ Best Practice https://bestpractice.bmj.com/topics/en-us/971.
Spotten LE, Corish CA, Lorton CM, Ui Dhuibhir PM, O'Donoghue NC, O’Connor B, Walsh TD (2017) Subjective and objective taste and smell changes in cancer. Ann Oncol 28:969–984. https://doi.org/10.1093/annonc/mdx018
Ui Dhuibhir P, Barrett M, O’Donoghue N, Gillham C, El Beltagi N, Walsh D (2020) Self-reported and objective taste and smell evaluation in treatment-naive solid tumour patients. Support Care Cancer 28:2389–2396. https://doi.org/10.1007/s00520-019-05017-3
Buttiron Webber T, Briata IM, DeCensi A, Cevasco I, Paleari L (2023) Taste and smell disorders in cancer treatment: results from an integrative rapid systematic review. Int J Mol Sci 24. https://doi.org/10.3390/ijms24032538
Gunn L, Gilbert J, Nenclares P, Soliman H, Newbold K, Bhide S, Wong KH, Harrington K, Nutting C (2021) Taste dysfunction following radiotherapy to the head and neck: a systematic review. Radiother Oncol 157:130–140. https://doi.org/10.1016/j.radonc.2021.01.021
Epstein JB, de Andrade ESSM, Epstein GL, Leal JHS, Barasch A, Smutzer G (2019) Taste disorders following cancer treatment: report of a case series. Support Care Cancer 27: 4587-4595. https://doi.org/https://doi.org/10.1007/s00520-019-04758-5
Ripamonti C, Fulfaro F (2010) Taste disturbance. In: Davies AN, Epstein JB (eds) Oral complications of cancer and its management. Oxford University Press, Oxford, pp 225–232
Spencer AS, da Silva DD, Capelas ML, Pimentel F, Santos T, Neves PM, Makitie A, Ravasco P (2021) Managing severe dysgeusia and dysosmia in lung cancer patients: a systematic scoping review. Front Oncol 11:774081. https://doi.org/10.3389/fonc.2021.774081
Pellegrini M, Merlo FD, Agnello E, Monge T, Devecchi A, Casalone V, Montemurro F, Ghigo E, Sapino A, Bo S (2023) Dysgeusia in patients with breast cancer treated with chemotherapy-a narrative review. Nutrients 15. https://doi.org/10.3390/nu15010226
Togni T, L, Mascitti M, Vignigni A, Alia S, Sartini D, Barlattani A, Emanuelli M, Santarelli A (2021) Treatment-related dysgeusia in oral and oropharyngeal cancer: a comprehensive review. Nutrients 13. https://doi.org/10.3390/nu13103325
Deshpande TS, Blanchard P, Wang L, Foote RL, Zhang X, Frank SJ (2018) Radiation-related alterations of taste function in patients with head and neck cancer: a systematic review. Curr Treat Options Oncol 19:72. https://doi.org/10.1007/s11864-018-0580-7
Twycross RG, Lack SA (1986) Control of alimentary symptoms in far advanced cancer. Churchill Livingstone, Edinburgh
Santini D, Armento G, Giusti R, Ferrara M, Moro C, Fulfaro F, Bossi P, Arena F, Ripamonti CI (2020) Management of orphan symptoms: ESMO Clinical Practice Guidelines for diagnosis and treatment. ESMO Open 5:e000933. https://doi.org/10.1136/esmoopen-2020-000933
Arksey H, O’Malley L (2005) Scoping studies: towards a methodological framework. Int. J. Soc. Res. Methodol 8:19–32. https://doi.org/10.1080/1364557032000119616
Peters MDJ, Marnie C, Tricco AC, Pollock D, Munn Z, Alexander L, McInerney P, Godfrey CM, Khalil H (2021) Updated methodological guidance for the conduct of scoping reviews. JBI Evid Implement 19:3–10. https://doi.org/10.11124/JBIES-20-00167
McGowan J, Straus S, Moher D, Langlois EV, O'Brien KK, Horsley T, Aldcroft A, Zarin W, Garitty CM, Hempel S, Lillie E, Tuncalp Ӧ, Tricco AC (2020) Reporting scoping reviews-PRISMA ScR extension. J Clin Epidemiol 123:177–179. https://doi.org/10.1016/j.jclinepi.2020.03.016
National Cancer Institute Dictionary of Cancer Terms (2022) https://www.cancer.gov/publications/dictionaries/cancerterms/def/advanced-cancer.
Aktas A, Walsh D, Hu B (2014) Cancer symptom clusters: an exploratory analysis of eight statistical techniques. J Pain Symptom Manage 48:1254–1266. https://doi.org/10.1016/j.jpainsymman.2014.02.006
Bovio G, Montagna G, Bariani C, Baiardi P (2009) Upper gastrointestinal symptoms in patients with advanced cancer: relationship to nutritional and performance status. Support Care Cancer 17:1317–1324. https://doi.org/10.1007/s00520-009-0590-x
Brisbois TD, de Kock IH, Watanabe SM, Baracos VE, Wismer WV (2011) Characterization of chemosensory alterations in advanced cancer reveals specific chemosensory phenotypes impacting dietary intake and quality of life. J Pain Symptom Manage 41:673–683. https://doi.org/10.1016/j.jpainsymman.2010.06.022
Mahmoud FA, Aktas A, Walsh D, Hullihen B (2011) A pilot study of taste changes among hospice inpatients with advanced cancer. Am J Hosp Palliat Care 28:487–492. https://doi.org/10.1177/1049909111402187
Ozalp GS, Uysal N, Oguz G, Kocak N, Karaca S, Kadiogullari N (2017) Identification of symptom clusters in cancer patients at palliative care clinic. Asia Pac J Oncol Nurs 4:259–264. https://doi.org/10.4103/apjon.apjon_17_17
Sweeney MP, Bagg J, Baxter WP, Aitchison TC (1998) Oral disease in terminally ill cancer patients with xerostomia. Oral Oncol 34:123–126. https://doi.org/10.1016/s1368-8375(97)00076-6
Tebidze N, Chikhladze NJ, E. Margvelashvili, V. Jincharadze, M. , Kordzaia D (2019) Perception of oral problems in patients with advanced cancer. Georgian Med News:50–56
Alsirafy SA, Abd El-Aal HH, Farag DE, Radwan RH, El-Sherief WA, Fawzy R (2016) High symptom burden among patients with newly diagnosed incurable cancer in a developing country. J Pain Symptom Manage 51:e1–e5. https://doi.org/10.1016/j.jpainsymman.2016.02.003
Alt-Epping B, Nejad RK, Jung K, Gross U, Nauck F (2012) Symptoms of the oral cavity and their association with local microbiological and clinical findings--a prospective survey in palliative care. Support Care Cancer 20:531–537. https://doi.org/10.1007/s00520-011-1114-z
Chaiviboontham SVH, McCorkle S, R. (2011) Symptom clusters in Thais with advanced cancer. Pac Rim Int J Nurs Res 14:265–277
Davies A, Buchanan A, Todd J, Gregory A, Batsari KM (2021) Oral symptoms in patients with advanced cancer: an observational study using a novel oral symptom assessment scale. Support Care Cancer 29:4357–4364. https://doi.org/10.1007/s00520-020-05903-1
Davies AN (2000) An investigation into the relationship between salivary gland hypofunction and oral health problems in patients with advanced cancer. Dissertation,. Kings College London
Davies AN, Kaur K (1998) Taste problems in patients with advanced cancer. Palliative Medicine 12:482–483
Hutton JL, Baracos VE, Wismer WV (2007) Chemosensory dysfunction is a primary factor in the evolution of declining nutritional status and quality of life in patients with advanced cancer. J Pain Symptom Manage 33:156–165. https://doi.org/10.1016/j.jpainsymman.2006.07.017
Kirkova J, Walsh D, Rybicki L, Davis MP, Aktas A, Tao J, Homsi J (2010) Symptom severity and distress in advanced cancer. Palliat Med 24:330–339. https://doi.org/10.1177/0269216309356380
Kirkova J, Aktas A, Walsh D, Rybicki L, Davis MP (2010) Consistency of symptom clusters in advanced cancer. Am J Hosp Palliat Care 27:342–346. https://doi.org/10.1177/1049909110369869
McGettigan N, Dhuibhir PU, Barrett M, Sui J, Balding L, Higgins S, O’Leary N, Kennedy A, Walsh D (2019) Subjective and objective assessment of taste and smell sensation in advanced cancer. Am J Hosp Palliat Care 36:688–696. https://doi.org/10.1177/1049909119832836
O’Donoghue A, Barrett M, Dhuibhir PU, Kennedy A, O’Leary N, Walsh D (2023) Taste and smell abnormalities in advanced cancer: negative impact on subjective food intake. Nutr Clin Pract 1:1–10. https://doi.org/10.1002/ncp.10943
Spichiger E, Muller-Frohlich C, Denhaerynck K, Stoll H, Hantikainen V, Dodd M (2011) Symptom prevalence and changes of symptoms over ten days in hospitalized patients with advanced cancer: a descriptive study. Eur J Oncol Nurs 15:95–102. https://doi.org/10.1016/j.ejon.2010.06.005
Tranmer JE, Heyland D, Dudgeon D, Groll D, Squires-Graham M, Coulson K (2003) Measuring the symptom experience of seriously ill cancer and noncancer hospitalized patients near the end of life with the memorial symptom assessment scale. J Pain Symptom Manage 25:420–429. https://doi.org/10.1016/s0885-3924(03)00074-5
Tsai JS, Wu CH, Chiu TY, Chen CY (2010) Significance of symptom clustering in palliative care of advanced cancer patients. J Pain Symptom Manage 39:655–662. https://doi.org/10.1016/j.jpainsymman.2009.09.005
Van Lancker A, Beeckman D, Van Den Noortgate N, Verhaeghe S, Van Hecke A (2017) Frequency and intensity of symptoms and treatment interventions in hospitalized older palliative cancer patients: a multicentre cross-sectional study. J Adv Nurs 73:1455–1466. https://doi.org/10.1111/jan.13230
Walsh D, Rybicki L (2006) Symptom clustering in advanced cancer. Support Care Cancer 14:831–836. https://doi.org/10.1007/s00520-005-0899-z
Webber K, Davies AN, Leach C, Waghorn M (2021) Symptom prevalence and severity in palliative cancer medicine. BMJ Support Palliat Care 0:1–3. https://doi.org/10.1136/bmjspcare-2020-002357
Webber K, Davies AN (2011) Validity of the memorial symptom assessment scale-short form psychological subscales in advanced cancer patients. J Pain Symptom Manage 42:761–767. https://doi.org/10.1016/j.jpainsymman.2011.02.007
Heald A, Pieper CF, Schiffman SS (1998) Taste and smell complaints in HIV-infected patients. AIDS 12:1667–1674. https://doi.org/10.1097/00002030-199813000-00015
Chang VT, Hwang SS, Feuerman M, Kasimis BS, Thaler HT (2000) The memorial symptom assessment scale short form (MSAS-SF). Cancer 89:1162–1171. https://doi.org/10.1002/1097-0142
Van Lancker A, Beeckman D, Verhaeghe S, Van Den Noortgate N, Grypdonck M, Van Hecke A (2016) An instrument to collect data on frequency and intensity of symptoms in older palliative cancer patients: a development and validation study. Eur J Oncol Nurs 21:38–47. https://doi.org/10.1016/j.ejon.2015.11.003
Henkin RI, Schechter PJ, Hoye R, Mattern CFT (1971) Idiopathic hypogeusia with dysgeusia, hyposmia, and dysosmia. A new syndrome. JAMA 217:434–440
Portenoy RK, Thaler HT, Kornblith AB, McCarthy Lepore J, Friedlander-Klar H, Kiyasu E, Sobel K, Coyle N, Kemeny N, Norton L, Scher H (1994) The Memorial Symptom Assessment Scale: an instrument for the evaluation of symptom prevalence, characteristics, and distress. Eur J Cancer 30:1226–1236. https://doi.org/10.1016/0959-8049(94)90182-1
Epstein JB, Smutzer G, Doty RL (2016) Understanding the impact of taste changes in oncology care. Support Care Cancer 24:1917–1931. https://doi.org/10.1007/s00520-016-3083-8
Enriquez-Fernandez BE, Martinez-Michel L, Thorlakson J, Wismer WV (2020) Patient-reported taste change assessment questionnaires used in the oncology setting: a narrative review. Eur J Oncol Nurs 47:101775. https://doi.org/10.1016/j.ejon.2020.101775
Walsh D, Donnelly S, Rybicki L (2000) The symptoms of advanced cancer: relationship to age, gender, and performance status in 1,000 patients. Support Care Cancer 8:175–179. https://doi.org/10.1007/s005200050281
Davies AN, Broadley K, Beighton D (2001) Xerostomia in patients with advanced cancer. J Pain Symptom Manage 22:820–825. https://doi.org/10.1016/s0885-3924(01)00318-9
Dong ST, Butow PN, Costa DS, Lovell MR, Agar M (2014) Symptom clusters in patients with advanced cancer: a systematic review of observational studies. J Pain Symptom Manage 48:411–450. https://doi.org/10.1016/j.jpainsymman.2013.10.027
DeWys WD, Walters K (1975) Abnormalities of taste sensation in cancer patients. Cancer 36:1888–1896. https://doi.org/10.1002/10970142
Rydholm M, Strang P (2002) Physical and psychosocial impact of xerostomia in palliative cancer care: a qualitative interview study. Int J Palliat Nurs 8:318–323. https://doi.org/10.12968/ijpn.2002.8.7.10671
Hopkinson JB (2007) How people with advanced cancer manage changing eating habits. J Adv Nurs 59:454–462. https://doi.org/10.1111/j.1365-2648.2007.04283.x
Zhou T, Yang K, Thapa S, Liu H, Wang B, Yu S (2017) Differences in symptom burden among cancer patients with different stages of cachexia. J Pain Symptom Manage 53:919–926. https://doi.org/10.1016/j.jpainsymman.2016.12.325
Jones JA, Chavarri-Guerra Y, Correa LBC, Dean DR, Epstein JB, Fregnani ER, Lee J, Matsuda Y, Mercadante V, Monsen RE, Rajimakers NJH, Saunders D, Soto-Perez-de-Celis E, Sousa MS, Tonkaboni A, Vissink A, Yeoh KS, Davies AN (2022) MASCC/ISOO expert opinion on the management of oral problems in patients with advanced cancer. Support Care Cancer 30:8761–8773. https://doi.org/10.1007/s00520-022-07211-2
Alderman B, Allan L, Amano K, Bouleuc C, Davis M, Lister-Flynn S, Mukhopadhyay S, Davies A (2022) Multinational Association of Supportive Care in Cancer (MASCC) expert opinion/guidance on the use of clinically assisted nutrition in patients with advanced cancer. Support Care Cancer 30:2983–2992. https://doi.org/10.1007/s00520-021-06613-y
Acknowledgements
We thank Fiona Lawlor, Health Sciences Librarian, Our Lady’s Hospice and Care Services and Diarmuid Stokes, Health Sciences Librarian University College Dublin, for their guidance and assistance during the literature search.
Funding
Open Access funding provided by the IReL Consortium MH is a PhD student and is co-funded by Our Lady’s Hospice and Care Services and Professor Davies’s research account.
Author information
Authors and Affiliations
Contributions
The first draft of the manuscript was written by MH with support from AD, and all the authors commented on and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix. MEDLINE search strategy
Appendix. MEDLINE search strategy
-
1.
Taste — MESH
-
2.
Taste alteration — keyword
-
3.
Taste change — keyword
-
4.
Taste disorders — MESH
-
5.
Taste distortion — keyword
-
6.
Taste disturbance — keyword
-
7.
Taste dysfunction — keyword
-
8.
Ageusia — MESH
-
9.
Dysgeusia — MESH
-
10.
Hypergeusia — keyword
-
11.
Hypogeusia — keyword
-
12.
Phantogeusia — keyword
-
13.
Chemosensory problems — keyword
-
14.
Altered taste — keyword
-
15.
Bad taste — keyword
-
16.
Lack of taste — keyword
-
17.
Metallic taste — keyword
-
18.
Taste perception — MESH
-
19.
Taste threshold — MESH
-
20.
Oral symptoms — keyword
-
21.
Oral conditions — keyword
-
22.
Oral diseases — keyword
-
23.
Oral problems — keyword
-
24.
Oral health — MESH
-
25.
Mouth diseases — MESH
-
26.
[1 or (2 to 25)]
-
27.
Neoplasms — MESH
-
28.
Cancer — keyword
-
29.
[27 or 28]
-
30.
[26 and 29]
-
31.
Palliative care — MESH
-
32.
Terminal care — MESH
-
33.
End of life care — keyword
-
34.
[31 or 32 or 33]
-
35.
[26 and 34]
-
36.
[35 not 30]
-
37.
[30 or 36]
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hannon, M., Shaw, A., Connolly, M. et al. Taste disturbance in patients with advanced cancer: a scoping review of clinical features and complications. Support Care Cancer 31, 562 (2023). https://doi.org/10.1007/s00520-023-08012-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00520-023-08012-x