Abstract
Purpose
Many partners of incurably ill cancer patients experience caregiver burden. The eHealth application “Oncokompas” supports these partners to manage their caregiver needs and to find optimal supportive care for themselves. The aim of this randomized controlled trial (RCT) was to investigate the reach of Oncokompas and its efficacy on caregiver burden, self-efficacy, and health-related quality of life (HRQOL).
Methods
The reach was estimated based on eligibility, participation rate, and an evaluation of the recruitment process. Efficacy on caregiver burden was measured using the Caregiver Strain Index + (CSI +). Secondary outcomes were self-efficacy (General Self-Efficacy Scale (GSE)) and HRQOL (EQ-5D VAS). Assessments were scheduled at baseline, 2 weeks after randomization and 3 months after baseline. Linear mixed models were used to compare longitudinal changes between the experimental and control group from baseline to the 3-month follow-up.
Results
The reach, in terms of eligibility and participation rate, was estimated at 83–91%. Partners were most likely reached via palliative care consultants, patient organizations, and palliative care networks. In the one-and-a-half-year recruitment period and via the 101 organizations involved, 58 partners were included. There were no significant effects of Oncokompas on caregiver burden, self-efficacy, or HRQOL.
Conclusion
The reach of Oncokompas among interested individuals was high, but the difficulties that were encountered to include partners suggest that the reach in real life may be lower. This study showed no effect of Oncokompas on caregiver burden, self-efficacy, or HRQOL in partners of incurably ill cancer patients.
Relevance
The results of this study may be used in the process of developing, efficacy testing, and implementing eHealth applications for caregivers of incurably ill cancer patients.
Trial registration
Netherlands Trial Register identifier: NTR7636/NL7411. Registered on November 23, 2018 (https://www.trialregister.nl/).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There is convincing evidence that informal caregiving for an incurably ill cancer patient is associated with physical, psychological, and social problems and that these problems negatively impact aspects of health-related quality of life (HRQOL) of informal caregivers [1,2,3,4,5,6,7,8,9,10,11,12,13,14]. Caregiver burden can be defined as “a multidimensional biopsychosocial reaction resulting from an imbalance of care demands relative to caregivers’ personal time, social roles, physical and emotional states, financial resources, and formal care resources given the other multiple roles they fulfill” [15]. There is a growing interest in healthcare resources to support informal caregivers of incurably ill patients. Many informal caregivers do not use these healthcare options. For instance, because they are unaware or unconcerned that their own HRQOL is being compromised, they are unaware of the available healthcare resources, they do not have access to these resources at the moment they need them, or they may feel that focusing at their own needs is at the expense of the patients’ needs [16,17,18,19,20,21]. Delivering interventions through the Internet may help to reach a greater number of informal caregivers [17, 22,23,24].
The eHealth self-management application Oncokompas was developed to support partners of incurably ill cancer patients to adopt an active role in improving their own HRQOL and to find optimal supportive care if needed. Oncokompas specifically targets partners of incurably ill cancer patients to optimally tailor information, advice, and supportive care options to their situation. Oncokompas helps partners of incurably ill cancer patients to monitor their own HRQOL using patient-reported outcome measures (PROMs), followed by automatically generated tailored feedback, self-care advice, and advice on supportive care services. The application is tailored to the partner’s personal characteristics and preferences and can be accessed 24/7 [25].
The aim of this randomized controlled trial (RCT) was to investigate the reach and efficacy of Oncokompas as a digital self-management instrument on caregiver burden, self-efficacy, and HRQOL among partners of patients with incurable cancer. The reach and efficacy were evaluated in the context of the RE-AIM model [26]. The reach was estimated based on eligibility, participation rate, and an evaluation of the recruitment process of this RCT. It is expected that Oncokompas reaches about 45% of the partners and that using Oncokompas helps partners to reduce caregiver burden and to increase self-efficacy and HRQOL [27, 28].
Methods
Study design
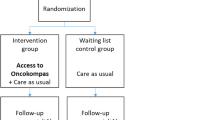
A prospective RCT with two parallel groups was conducted to investigate the reach and efficacy of Oncokompas among partners of patients with incurable cancer. Partners in the intervention group got access to Oncokompas directly after completing the baseline questionnaire, and partners in the control group got access after three months (i.e., after completing the last questionnaire). Outcome measures were collected at baseline (t0), 2 weeks after randomization (t1), and 3 months after the baseline measurement (t2).
The study protocol was approved by the Medical Ethics Committee of VU University Medical Center (2018.517). This trial was registered in the Netherlands Trial Register (NTR7636/NL7411), and the study protocol was published previously [25]. All participants provided written informed consent. The CONSORT guidelines (CONsolidated Standards of Reporting Trials) were used to report on this trial [29].
Study population
Inclusion criteria were as follows: being an adult (aged ≥ 18 years) partner of an incurably ill cancer patient and having access to an e-mail address. Partners self-identified as partner. There were no restrictions with regard to their marital status, living situation, duration, or quality of their relationship. Patients were defined as incurably ill if their partner reported that the patient did not have curative treatment options. Partners were excluded when they had severe cognitive impairments or when they had a poor understanding of the Dutch language. They were also excluded when their partner with cancer already used Oncokompas for patients with incurable cancer.
Recruitment
A multi-component recruitment strategy was followed in which healthcare professionals in various settings were asked to place and spread recruitment materials and to inform partners of incurably ill cancer patients on the study (Table 1). Partners could also contact the researchers directly by using the reply form at the Oncokompas website or by e-mailing the researchers. Recruitment materials consisted of leaflets in waiting rooms and offices of healthcare providers, and online advertising on websites, newsletters, and social media. The contact details of the researcher and URL of the Oncokompas website (www.oncokompas.nl) were mentioned in all materials.
Study procedures
Individuals who expressed interest in participating in this study were contacted by a researcher to be further informed about the study. Eligible partners received an information letter and informed consent form. After signing informed consent, partners received the baseline questionnaire by e-mail. After completion of the baseline questionnaire, partners were randomly assigned to a study arm. Partners randomized to the intervention group received an invitation e-mail for Oncokompas to activate their personal account. Partners randomized to the control group received this e-mail after completion of the third questionnaire (t2).
Randomization
Partners were randomized in a 1:1 ratio. Block randomization was used with a random block length of four, six, or eight. Stratification was not applied. The randomization scheme was computer-generated, created by a researcher not involved in the study, who also performed the allocation of participants. Neither the researcher, and, because of the nature of the intervention, participants could not be blinded.
Wait list control group
All partners received care as usual during their participation in the wait list control group. Care as usual was defined as all care provided by healthcare professionals regardless of study participation. Care as usual may for example consist of consults with a medical specialist, general practitioner, psychotherapist, or physiotherapist.
Intervention
Oncokompas is an eHealth self-management application, consisting of three steps: measure, learn, and act (Fig. 1). Previously, a version of Oncokompas has been developed for cancer patients during or after curative treatment [27] and for incurably ill cancer patients [28]. All versions were developed using a stepwise, iterative, and participatory approach, actively involving end users and health care professionals [30]. The development process consisted of six steps: (1) selection of topics, (2) selection of PROMs, (3) determining cut-off scores, (4) drafting information texts, (5) drafting self-management advice texts, and (6) selecting health care options. This means that within the existing framework of Oncokompas, new content for partners of incurably ill patients was developed. Each step was carried out by researchers (AS, NH, and KH) and discussed with an expert team consisting of health care professionals, partners of incurably ill cancer patients, and researchers. Previously, two RCTs were conducted on the efficacy of Oncokompas among cancer survivors [27] and incurably ill cancer patients [28] that did not show an effect at patient activation (primary outcome), but did show effects on HRQOL and specific symptoms among survivors.
In the first step of Oncokompas, “measure,” partners complete a questionnaire on their personal characteristics, used to automatically display the topics appropriate for this individual (e.g., when someone is retired, the topic about “work” will not be shown). Then, partners can select which topics they want to address within Oncokompas (e.g., fatigue, loneliness, or financial problems). The topics target four domains of quality of life: physical, psychological and social functioning, and existential issues. Subsequently, PROMs are used to measure partners’ functioning on the selected topics [25]. In the step, “learn”, Oncokompas provides information and feedback on partners’ outcomes, tailored to their personal characteristics and preferences. Using a traffic-light system (green, orange, and red), partners get an overview of their well-being per topic. A green score means that the partner is doing well on this topic, an orange score means that this topic could use attention and support, and a red score means that this topic needs attention and support. Then, Oncokompas provides comprehensive self-care advice. Lastly, within the step “act,” partners receive a personal overview of supportive care options for themselves, with options for professional guidance when needed.
Study measures
Caregiver burden was assessed using the Caregiver Strain Index + (CSI +). The CSI + measures the self-reported burden that informal caregivers experience as a result of caring for their loved ones (13 items), as well as positive and rewarding experiences as a result of informal caregiving (5 items). Response options are “yes” (coded as 1 for the negative items and − 1 for the positive items) and “no” (always coded as 0). The total score range is − 5 to 13. A higher score indicates more caregiver burden. The CSI + does not have a cut-off score. The total CSI + score was analyzed, as well as the negative and positive items separately. In the separate analyses, a higher score indicates more caregiver burden. In contrary to the way positive items of the CSI + were coded (as − 1), in the separate analyses, positive items were coded as 1, so that a higher score indicates a more positive caregiver experience [31,32).
Self-efficacy was assessed using the General Self-Efficacy Scale (GSE). The GSE is a 10-item self-report questionnaire, assessing how a person deals with difficult situations in life. The items have a 4-point Likert scale, ranging from 1 (not at all true) up to 4 (exactly true). The total score is calculated by adding up the scores on the 10 items and ranges from 10 to 40. A higher score indicates a greater sense of self-efficacy. The GSE does not have a cut-off score. The international average GSE score in the general population is 29.55 [33, 34].
HRQOL was measured using the visual analogue scale (VAS) of the self-report questionnaire EuroQol-5D (EQ-5D). The VAS ranges from 0 to 100, in which 100 indicates the best imaginable health state. The norm score of general Dutch citizens from 55 to 64 years is 80.7 [35, 36].
Partners’ and patients’ sociodemographic and health-related characteristics were assessed at baseline using a study specific questionnaire.
Sample size
To demonstrate an increase on the CSI + of at least 0.5 standard deviations in the intervention group compared to the control group (i.e., between group change of 0.5 SD) between t0 and t2 as statistically significant in a one-tailed test using a significance level of 5% (alpha = 0.05) and a power of 80% (1 − β = 0.80), 51 participants were required at t2 in each study arm. Anticipating a drop-out rate of 25% between t0 and t2, 68 participants per study arm needed to be included at t0. In total, a sample of 136 participants was needed.
Statistical analyses
Reach was estimated based on eligibility and participation rate. Eligibility rate was calculated as the number of eligible partners divided by the number of partners who were informed on the study after they expressed interest in Oncokompas. The participation rate was calculated by dividing the number of included partners by the number of eligible partners.
Descriptive statistics were used to describe the recruitment process, sociodemographic and health-related characteristics of the partner and the patient, and the outcome measures at baseline.
Linear mixed models (LMM) were used to compare longitudinal changes in primary and secondary outcome measures in both study arms between t0, t1, and t2. Fixed effects were used for study arm, measurement, and their two-way interaction, and a random intercept for subjects. All analyses were conducted according to the intention-to-treat principle. Missing data was not imputed as LMM accounts for missing data. As sensitivity analyses, the LMM were repeated comparing participants who used Oncokompas as intended with participants in the control condition. Usage as intended was defined as completing the steps “measure” and “learn” at least for one topic.
All analyses were performed using the IBM Statistical Package for the Social Sciences (SPSS) version 27 (IBM Corp., Armonk, NY USA). A p-value of < 0.05 was considered significant for all analyses.
Results
Reach: recruitment process
During the recruitment process, 656 organizations involved in palliative care were asked to participate in recruiting partners (Table 1). In total, 101 agreed to participate (15%), 67 declined (10%), and the majority did not respond (n = 488, 74%). Main reasons for organizations that declined were the following: having no time, already being involved in other studies, or not having partners of incurably ill cancer patients in their care. The types of organization that most often agreed to participate, in terms of percentages, were palliative care consultants (100%), patient organizations (48%), palliative care networks (41%), and centers for supportive cancer care (38%). The types of organizations that were least likely to participate were home care organizations (2%), general practitioners (4%), and hospitals (11%). Despite all efforts, after recruiting for almost one and a half year, the inclusion of partners lagged behind considerably. Part of the study (March-August 2020) took place during the COVID-19 pandemic. Due to the national lockdown in the Netherlands, many organizations were not able to continue their services as they were used to. Reaching the target of 136 included partners was no longer considered feasible, and therefore, the study stopped in September 2020. The 101 parties involved in the recruitment process led to 93 individuals expressing interest in Oncokompas and 58 inclusions.
Reach: eligibility and participation rate
Between March 2019 and August 2020, 93 individuals expressed interest in Oncokompas. Sixteen of them could not be contacted. The other 77 were informed about the study, of which 64 were eligible (eligibility rate 83%). Reasons for ineligibility were as follows: patient (the partner’s partner) passed away (n = 6), patient was in the terminal phase of the disease (n = 2), patient still had options for curative treatment (n = 2), applicant was not the partner but another informal caregiver (n = 2), and having no computer (n = 1). Of the 64 eligible partners, 58 agreed to participate in the study (participation rate 91%). Reasons for not agreeing to participate were the following: not being interested (n = 3), participation being too confronting (n = 2), and having privacy concerns (n = 1). The flow diagram of the study is shown in Fig. 2.
Efficacy
Of the 58 included partners, 28 were allocated to the intervention group and 30 to the control group (Fig. 1). Mean age was 57 years, and two-thirds (67%) were female. The majority had children (86%), were highly educated (55%), and employed (60%). Almost half of the partners (45%) reported no comorbidities (Table 2). The patients that the partners were caring for were on average 59 years of age and their health (as perceived by the partner) was on average 4.7 on a scale of 0 to 10. Most of the patients had lung cancer (19%) or a brain tumor (17%), and received treatment primarily directed at the disease (as opposed to treatment primarily directed at reducing symptoms) (72%). Fifty-one percent were diagnosed with cancer more than 2 years ago (Table 3).
Results of the linear mixed model analyses are shown in Table 4. No significant difference was found in the course of caregiver burden (CSI +) in the intervention group, compared to the control group. The estimated difference in change from t0 to t2 was 0.3 points (90% CI − 0.8–1.5). This means that the estimated change in the intervention group (t0–t2) was 0.3 points higher than in the control group. The p-value of the interaction between the study arm and the time of assessment was 0.64.
Also, the course of caregiver burden (negative items of the CSI +), positive caregiving experience (positive items of the CSI +), self-efficacy (GSE), and HRQOL (EQ-5D VAS) did not differ significantly between partners randomized into the intervention or wait list control group.
Usage of Oncokompas
Of the 28 partners in the intervention group, 27 activated their account and 22 of them (81%) used Oncokompas as intended during the three-month follow-up period. The median number of logins among intended users was 3 (interquartile range (IQR) 2–4). The course of all outcome measures was not significantly different between partners who used Oncokompas as intended and partners in the control condition.
Discussion
This study examined the reach and efficacy of the eHealth self-management application Oncokompas, among partners of patients with incurable cancer. The reach was estimated at 83–91%. There was no significant effect on caregiver burden, self-efficacy, or HRQOL of the partners.
In this study, reach was defined as a combination of the eligibility and participation rate of individuals with an expressed interest in Oncokompas, complemented by an evaluation of the recruitment process. While the eligibility and participation rate were high, the difficulties that were encountered to include partners suggest that in real life the reach may be lower. In previous studies, the reach of Oncokompas was estimated to be 45–68% in cancer survivors [27] and 63% in patients with incurable cancer [28]. Main reasons for not reaching cancer survivors were the following: wanting to leave the period of being ill behind, no symptom burden, or lacking computer skills [37]. Main reasons for not reaching patients with incurable cancer were as follows: participation being too confronting or lacking computer skills [28]. In the present study, various online channels were used to recruit partners, which may explain that a lack of computer skills was not a main reason for not reaching the target population, and which may have overestimated the eligibility and participation rate. In contrast to the studies among cancer survivors and patients, where recruitment took place in hospitals solely, partners were most likely reached via palliative care consultants, palliative care networks, and patient organizations and less via hospitals. A hospital-based recruitment strategy might have been more successful, but was not feasible for the present study, because in parallel incurably ill cancer patients were recruited in the hospital for the study on the efficacy of Oncokompas for patients [28]. Unfortunately, during the present study, all recruitment channels were affected by the national lock-down due to the COVID-19 pandemic.
Based on the results of this study, it cannot be concluded that Oncokompas decreases caregiver burden, and increases self-efficacy or HRQOL in partners of incurably ill cancer patients. A meta-analysis suggests that caregiver burden is hardly affected by eHealth interventions [38]. The absence of significant effects of Oncokompas is in line with a recent study on the efficacy of the version of Oncokompas for incurably ill cancer patients [28], but not with a large study on the efficacy of the version of Oncokompas for cancer survivors [27]. Oncokompas for cancer survivors differs from the other two versions of Oncokompas, in that it has several tumor-specific modules and its effects were mainly found among tumor-specific symptoms. Oncokompas for partners is tailored to the characteristics and preferences of the partner, but may need to be further tailored to the specific demands of caring for a patient with a specific tumor type [39]. At the same time, Oncokompas may have had specific effects for partners, such as feeling less fatigued or lonely, but these specific effects may not have been captured by the generic outcome measures used in this study. The absence of significant effects of Oncokompas may also be explained by the low number of logins among the 81% who used Oncokompas as intended (median number of logins = 3) or by the finding in a previous study that about 60% of the Oncokompas users feel that the tailored content is still not applicable to their situation [37].
There are also some limitations that may explain these results. First, the sample size was smaller than intended, and therefore, the power to detect changes in the outcome measures was insufficient. Second, Oncokompas was developed before the COVID-19 pandemic, but the RCT was largely conducted during the pandemic. There might have been an effect on the personalized supportive care options as provided in the third step within Oncokompas (the “act” component). When a user has a red score on a topic, the feedback always included the advice to contact a healthcare professional. These contacts may have changed from face-to-face contact to contact through telehealth during the COVID-19 pandemic. Third, the follow-up period of three months might have been too short to measure effects that take more than three months (e.g., psychotherapy or physiotherapy). A fourth explanation could be that 51% of the participants were caring for a patient diagnosed more than two years ago. These partners may have already learned to cope with the situation and did not need Oncokompas at that moment anymore. In any case, it may be better to provide access to Oncokompas at an early stage, for instance, shortly after a diagnosis of incurable cancer. Such an approach would also fit well into advanced cancer care planning [40].
Alongside this RCT, a cost-utility analysis was planned. It was expected that Oncokompas would improve quality adjusted life years at acceptable costs, compared to the wait list control group [25]. Because the sample size was smaller than expected, this cost-utility analysis was deemed not to be feasible and was not carried out. Nonetheless, this project generated new knowledge on the reach and efficacy of an eHealth self-management intervention among partners of incurably ill cancer patients.
In conclusion, the reach of Oncokompas among interested individuals was high, but the difficulties that were encountered to include partners suggest that the reach in real life may be lower. This study showed no effect of Oncokompas on caregiver burden, self-efficacy, or HRQOL in partners of incurably ill cancer patients.
IMVdL, KH, and AS developed the eHealth self-management application Oncokompas and were involved in the design of the study. AS, KH, and IMVdL coordinated the study and were responsible for all aspects of local organization which they discussed in monthly meetings. IMVdL was the leading researcher responsible for the trial, overseeing conduct and progress. AS and NH were responsible for day-to-day support of the trial and aware of the outcome of the allocation during randomization. MR, KH, and BLW performed the data analyses. AS, MR, KH, and IMVdL drafted the manuscript. NH, FJ, BLW, and PC critically revised the manuscript. All authors read and approved the final manuscript.
Data availability
The datasets analyzed during the current study will be available in the EASY repository of DANS-KNAW after completion of the thesis that will be written reserving the generated data. The (intellectual) property rights with regard to the generated data will reside at the Vrije Universiteit Amsterdam. Interested parties can request a non-exclusive license for research and educational purposes. The data will be available up to 15 years after the end of the study.
Abbreviations
- CSI :
-
Caregiver Strain Index
- EQ-5D :
-
EuroQol 5-Dimensions questionnaire
- GSE :
-
General Self-Efficacy
- HRQOL :
-
Health-related quality of life
- LMM :
-
Linear mixed models
- PROMs :
-
Patient-reported outcome measures
- RCT :
-
Randomized controlled trial
- CONSORT :
-
CONsolidated Standards of Reporting Trials
References
Given BA, Given CW, Kozachik S (2001) Family support in advanced cancer. CA Cancer J Clin 51(4):213–31. https://doi.org/10.3322/canjclin.51.4.213
Geng H, Chuang D, Yang F, Yang Y, Liu W, Liu L et al (2018) Prevalence and determinants of depression in caregivers of cancer patients: a systematic review and meta-analysis. Medicine (United States) 97(39):e11863. https://doi.org/10.1097/MD.0000000000011863
Shaffer KM, Jacobs JM, Nipp RD, Carr A, Jackson VA, Park ER et al (2017) Mental and physical health correlates among family caregivers of patients with newly-diagnosed incurable cancer: a hierarchical linear regression analysis. Support Care Cancer 25(3):965–971. https://doi.org/10.1007/s00520-016-3488-4
van Roij J, Brom L, Youssef-El Soud M, van de Poll-Franse L, Raijmakers NJH (2019) Social consequences of advanced cancer in patients and their informal caregivers: a qualitative study. Support Care Cancer 27(4):1187–95. https://doi.org/10.1007/s00520-018-4437-1
van Roij J, Brom L, Sommeijer D, van de Poll-Franse L, Raijmakers N (2021) Self-care, resilience, and caregiver burden in relatives of patients with advanced cancer: results from the eQuiPe study. Support Care Cancer 29(12):7975–7984. https://doi.org/10.1007/s00520-021-06365-9
Weaver R, O’Connor M, Golding RM, Gibson C, White R, Jackson M et al (2022) “My life’s not my own”: a qualitative study into the expectations of head and neck cancer carers. Support Care Cancer 30(5):4073–4080. https://doi.org/10.1007/s00520-021-06761-1
Young J, Snowden A (2017) A systematic review on the factors associated with positive experiences in carers of someone with cancer. Eur J Cancer Care 26(3):e12544. https://doi.org/10.1111/ecc.12544
Pitceathly C, Maguire P (2003) The psychological impact of cancer on patients’ partners and other key relatives: a review. Eur J Cancer 39(11):1517–1524. https://linkinghub.elsevier.com/retrieve/pii/S0959804903003095
Braun M, Mikulincer M, Rydall A, Walsh A, Rodin G (2007) Hidden morbidity in cancer: spouse caregivers. J Clin Oncol 25(30):4829–34. https://doi.org/10.1200/JCO.2006.10.0909
Stenberg U, Ruland CM, Miaskowski C (2010) Review of the literature on the effects of caring for a patient with cancer. Psychooncology 19(10):1013–1025. https://doi.org/10.1002/pon.1670
Mazanec SR, Daly BJ, Douglas SL, Lipson AR (2011) Work productivity and health of informal caregivers of persons with advanced cancer. Res Nurs Heal 34(6):483–495. https://doi.org/10.1002/nur.20461
Bevans M, Sternberg EM (2012) Caregiving burden, stress, and health effects. JAMA 307(4):398–403
Wadhwa D, Burman D, Swami N, Rodin G, Lo C, Zimmermann C (2013) Quality of life and mental health in caregivers of outpatients with advanced cancer. Psychooncology 22(2):403–410. https://doi.org/10.1002/pon.2104
Peters MEWJ, Goedendorp MM, Verhagen SAHHVM, Smilde TJ, Bleijenberg G, Van Der Graaf WTA (2015) A prospective analysis on fatigue and experienced burden in informal caregivers of cancer patients during cancer treatment in the palliative phase. Acta Oncol 54(4):500–506. https://doi.org/10.3109/0284186X.2014.953254
Given B, Kozachik S, Collins C et al (2001) Caregiver role strain. In: Nursing care of older adults diagnosis: Outcome and interventions. Mosby, St. Louis
Lambert SD, Harrison JD, Smith E, Bonevski B, Carey M, Lawsin C et al (2012) The unmet needs of partners and caregivers of adults diagnosed with cancer: a systematic review. BMJ Support Palliat Care 2(3):224–230. https://doi.org/10.1136/bmjspcare-2012-000226
Applebaum AJ, Breitbart W (2013) Care for the cancer caregiver: a systematic review. Palliat Support Care 11(3):231–252. https://doi.org/10.1017/S1478951512000594
Sklenarova H, Krümpelmann A, Haun MW, Friederich HC, Huber J, Thomas M et al (2015) When do we need to care about the caregiver? Supportive care needs, anxiety, and depression among informal caregivers of patients with cancer and cancer survivors. Cancer 121(9):1513–1519. https://doi.org/10.1002/cncr.29223
Zavagli V, Raccichini M, Ostan R, Ercolani G, Franchini L, Varani S et al (2022) Identifying the prevalence of unmet supportive care needs among family caregivers of cancer patients: an Italian investigation on home palliative care setting. Support Care Cancer 30(4):3451–3461. https://doi.org/10.1007/s00520-021-06655-2
Wang T, Molassiotis A, Chung BPM, Tan JY (2018) Unmet care needs of advanced cancer patients and their informal caregivers: a systematic review. BMC Palliat Care 17(1):1–29. https://doi.org/10.1186/s12904-018-0346-9
Dionne-Odom JN, Applebaum AJ, Ornstein KA, Azuero A, Warren PP, Taylor RA et al (2018) Participation and interest in support services among family caregivers of older adults with cancer. Psychooncology 27(3):969–976. https://doi.org/10.1002/pon.4603
Slev VN, Mistiaen P, Pasman HRW, de Leeuw IMV, Uden-Kraan CF, va., Francke AL, (2016) Effects of eHealth for patients and informal caregivers confronted with cancer: a meta-review. Int J Med Inform 87:54–67. https://doi.org/10.1016/j.ijmedinf.2015.12.013
Köhle N, Drossaert CHC, Van Uden-Kraan CF, Schreurs KMG, Hagedoorn M, Verdonck-de Leeuw IM et al (2018) Intent to use a web-based psychological intervention for partners of cancer patients: associated factors and preferences. J Psychosoc Oncol 36(2):203–221. https://doi.org/10.1080/07347332.2017.1397831
Su Z, Li X, McDonnell D, Fernandez AA, Flores BE, Wang J (2021) Technology-based interventions for cancer caregivers: concept analysis. JMIR Cancer 7(4):e22140. https://cancer.jmir.org/2021/4/e22140
Schuit AS, Holtmaat K, Hooghiemstra N, Jansen F, Lissenberg-Witte BI, Coupé VMH et al (2020) Efficacy and cost-utility of the eHealth self-management application “Oncokompas,” helping partners of patients with incurable cancer to identify their unmet supportive care needs and to take actions to meet their needs: a study protocol of a randomized controlled trial. Trials 21(1):124. https://doi.org/10.1186/s13063-019-4037-5
Glasgow RE, Vogt TM, Boles SM (1999) Evaluating the public health impact of health promotion interventions: the RE-AIM framework. Am J Public Health 89(9):1322–1327. https://doi.org/10.2105/AJPH.89.9.1322
van der Hout A, van Uden-Kraan CF, Holtmaat K, Jansen F, Lissenberg-Witte BI, Nieuwenhuijzen GAP et al (2020) Role of eHealth application Oncokompas in supporting self-management of symptoms and health-related quality of life in cancer survivors: a randomised, controlled trial. Lancet Oncol 21(1):80–94. https://linkinghub.elsevier.com/retrieve/pii/S1470204519306758
Schuit AS, Holtmaat K, Lissenberg-Witte BI, Eerenstein SEJ, Zijlstra JM, Eeltink C et al (2022) Efficacy of the eHealth application Oncokompas, facilitating incurably ill cancer patients to self-manage their palliative care needs: a randomized controlled trial. Lancet Reg Heal – Eur 18:100390. https://doi.org/10.1007/978-3-030-03874-8
Moher D, Hopewell S, Schulz KF, Montori V, Gotzsche PC, Devereaux PJ et al (2010) CONSORT 2010 Explanation and Elaboration: updated guidelines for reporting parallel group randomised trials. BMJ. 2010 Mar 23;340:c869–c869. https://doi.org/10.1136/bmj.c869
Van Gemert-Pijnen JEWC, Nijland N, Van Limburg M, Ossebaard HC, Kelders SM, Eysenbach G et al (2011) A holistic framework to improve the uptake and impact of ehealth technologies. J Med Internet Res 13(4):e1672. https://doi.org/10.2196/jmir.1672
Robinson BC (1983) Validation of a Caregiver Strain Index. J Gerontol 38(3):344–8. https://doi.org/10.1093/geronj/38.3.344
Al-Janabi H, Frew E, Brouwer W, Rappange D, Van Exel J (2010) The inclusion of positive aspects of caring in the Caregiver Strain Index: tests of feasibility and validity. Int J Nurs Stud 47(8):984–993. https://doi.org/10.1016/j.ijnurstu.2009.12.015
Schwarzer R, Jerusalem M, Weinman J, Wright S, Johnston M (1995) Generalised self-efficacy scale. Causal and control beliefs. In: Weinman J, Wright S, Johnston M (eds) Measures in health psychology: a user’s portfolio. NFER-Nelson, Windsor
Scholz U, Gutiérrez Doña B, Sud S, Schwarzer R (2002) Is general self-efficacy a universal construct? Psychometric findings from 25 countries. Eur J Psychol Assess 18(3):242–251. https://doi.org/10.1027//1015-5759.18.3.242
Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D et al (2011) Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 20(10):1727–1736. https://doi.org/10.1007/s11136-011-9903-x
Janssen B, Szende, A (2014) Population norms for the EQ-5D. In: Szende A, Janssen B, Cabases J (eds) Self-reported population health: an international perspective based on EQ-5D. Springer Netherlands, Dordrecht, p 19–30. https://doi.org/10.1007/978-94-007-7596-1
van der Hout A, van Uden-Kraan CF, Holtmaat K, Jansen F, Lissenberg-Witte BI, Nieuwenhuijzen GAP et al (2021) Reasons for not reaching or using web-based self-management applications, and the use and evaluation of Oncokompas among cancer survivors, in the context of a randomised controlled trial. Internet Interv 25:100429. https://linkinghub.elsevier.com/retrieve/pii/S2214782921000695
Li Y, Li J, Zhang Y, Ding Y, Hu X (2022) The effectiveness of e-Health interventions on caregiver burden, depression, and quality of life in informal caregivers of patients with cancer: a systematic review and meta-analysis of randomized controlled trials. Int J Nurs Stud 127:104179. https://doi.org/10.1016/j.ijnurstu.2022.104179
Schuit AS, Zwieten V, Holtmaat K, Cuijpers P, Eerenstein SEJ, Leemans CR et al (2021) Symptom monitoring in cancer and fully automated advice on supportive care: patients’ perspectives on self-management strategies and the eHealth self-management application Oncokompas. Eur J Cancer Care 30(6):1–13. https://doi.org/10.1111/ecc.13497
Brinkman- Stoppelenburg A, Rietjens JAC, Van Der Heide A (2014) The effects of advance care planning on end-of-life care: a systematic review. Palliat Med 28(8):1000–1025. https://doi.org/10.1177/0269216314526272
Funding
This research is supported by a grant from ZonMw, The Netherlands Organization for Health Research and Development (project number: 844001105). The study and its design were critically revised by ZonMw during the grant application process. ZonMw plays no part in the data collection, management, analysis and interpretation of the data, the writing of the report, and the decision to submit the report for publication.
Author information
Authors and Affiliations
Contributions
IMVdL, KH, and AS developed the eHealth self-management application Oncokompas and were involved in the design of the study. AS, KH, and IMVdL coordinated the study and were responsible for all aspects of local organization which they discussed in monthly meetings. IMVdL was the leading researcher responsible for the trial, overseeing conduct and progress. AS and NH were responsible for day-to-day support of the trial and aware of the outcome of the allocation during randomization. MR, KH and BLW performed the data analyses. AS, MR, KH, and IMVdL drafted the manuscript. NH, FJ, BLW and PC critically revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Research involving human participants
This study was conducted in accordance with the Declaration of Helsinki and local laws and regulations. The study protocol was approved by the Medical Ethical Committee of VU University Medical Center, Amsterdam, The Netherlands (2018.517).
Ethics approval
The study protocol was approved by the Medical Ethics Committee of VU University Medical Center (2018.517).
Consent to participate
Eligible partners were fully informed about the study and provided written informed consent before participating in the study. Interested partners received an information form about the study and were also informed by phone or in a face-to-face conversation with the researcher. The intervention investigated in this trial was an online self-management application with negligible risks. There was no compensation available for trial participation; partners participating in the study had free access to Oncokompas.
Competing interests
IMVdL is named as the inventor of Oncokompas, the self-management application that was examined in this study. IMVdL reports grants from the Netherlands Organization for Health Research and Development (ZonMw) (project number: 844001105) and the Dutch Cancer Society (KWF Kankerbestrijding) (project number: VUP2014-7202). The other authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Authors’ information
AS is a communication scientist. MR, PC, and KH are psychologist. NH is a movement scientist. FJ is a health scientist and epidemiologist. BLW is a biostatistician. IMVdL is a psychologist, speech therapist, and linguist.
The original version of this article was revised. The family name should be Verdonck-de Leeuw.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schuit, A.S., Rienks, M.M., Hooghiemstra, N. et al. Reach and efficacy of the eHealth application Oncokompas, facilitating partners of incurably ill cancer patients to self-manage their caregiver needs: a randomized controlled trial. Support Care Cancer 30, 10191–10201 (2022). https://doi.org/10.1007/s00520-022-07441-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-022-07441-4