Abstract
Background
To identify the association between diabetes mellitus (DM) and the risk of chemotherapy-induced peripheral neuropathy (CIPN) through a systematic review and meta-analysis.
Methods
An electronic literature search was conducted in PubMed, Embase, Web of Science, the Wanfang database, the VIP Journals database (CQVIP), the China National Knowledge Infrastructure (CNKI) database, and the China Biology Medicine database (Sinomed) between January 2010 and January 2021. Articles were included if they investigated CIPN and DM. Stata 15.1 was used to analyze the data.
Results
We examined 8923 cancer patients from 25 studies comprising 9 cohort studies and 16 case–control studies. Meta-analysis showed that there was a statistically significant positive correlation between DM and CIPN (odds ratio [OR] = 1.60, 95% confidence interval [CI] = 1.38–1.85, P < 0.001). Egger’s test (P = 0.824) showed no evidence of publication bias. The positive associations did not significant differ by study type, study quality, evaluation instrument, and type of antineoplastic drug. Omission of any single study had little effect on the combined risk estimate. Little evidence of heterogeneity was observed.
Conclusion
This meta-analysis provides evidence of a significant positive association between DM and risk of CIPN. Furthermore, a more detailed evaluation is warranted for cancer patients with diabetes when they are treated with antineoplastic drugs that have the potential to cause peripheral neuropathy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With the gradual prolongation of the survival time of cancer patients, attention should increasingly focus on the long-term toxicity associated with cancer treatment because of its potential to affect the quality of life of cancer patients [1]. Chemotherapy-induced peripheral neuropathy (CIPN), which can lead to permanent symptoms and disability in cancer survivors, is a prominent complication associated with this long-term toxicity [2]. CIPN is a frequent side effect of several commonly used antineoplastic agents, including platinum-based drugs (cisplatin, carboplatin, and oxaliplatin), taxanes (paclitaxel and docetaxel), vincristine, and eribulin, all of which are widely used as therapies for a variety of cancers [3]. Studies have shown that the incidence of CIPN ranges from 19 to more than 85% [4, 5]. Long-term follow-up results suggest that CIPN symptoms may persist for several years or even a lifetime after the cessation of chemotherapy, seriously affecting the quality of life of patients [6].
The most common symptoms of CIPN are sensory symptoms, such as pain, numbness, and tingling. However, some patients may have difficulties in fine motor coordination, sensory ataxia, and autonomic dysfunction [7]. Although numerous clinical studies have been conducted on the prevention and treatment of CIPN, none has provided conclusive evidence for a clinically beneficial agent in the treatment of CIPN, except for duloxetine, which is currently recommended for the treatment of painful neuropathy [8]. Therefore, understanding the risk factors for this side effect of chemotherapy is critical for preventing severe CIPN and may help guide further research and treatment. Recent studies have shown that drug cumulative dose is the most important influencing factor and accurate predictor of all CIPN [7, 9]. Other possible factors include the duration of drug infusion, baseline neuropathy, age, sex, smoking history, renal dysfunction (low creatinine clearance rate), metabolic-lifestyle factors, and genetic predisposition [7, 9,10,11,12,13].
Diabetes mellitus (DM) is one of the most important, chronic, noncommunicable diseases worldwide. Many cancer patients have a history of DM. Diabetic peripheral neuropathy (DPN) is a common chronic complication in diabetic patients and its clinical manifestations are similar to those of CIPN. The relationship between DM and CIPN is controversial. Although preexisting DPN is considered to be a risk factor for CIPN, it is unclear whether the incidence and severity of the latter are greater in patients with diabetes who do not have peripheral neuropathy symptoms at baseline when they receive chemotherapy [14]. Therefore, we conducted a meta-analysis to quantitatively evaluate the association between CIPN and DM among cancer patients.
Methods
Search strategy
We conducted a comprehensive search in PubMed, Embase, and Web of Science (WOS). Chinese databases, including the Wanfang database, VIP Journals database (CQVIP), and China National Knowledge Infrastructure (CNKI) database, were also searched in order to expand the scope of retrieval. We obtained all studies published between January 2010 and January 2021 that reported on DM and CIPN using the Medical Subject Headings (MeSH) terms “chemotherapy” or the text word terms “antineoplastic agents,” “oxaliplatin,” “paclitaxel,” “docetaxel,” “vincristine,” “bortezomib,” “thalidomide,” or “platinum”; the MeSH term “diabetes mellitus” or the text word terms “diabetes complications,” “IDDM,” “NIDDM,” “MODY,” “T1DM,” or “T2DM”; and the MeSH term “neurotoxicity” or the text word terms “neuropathy,” “neuropathic,” or “nerve.” Multiple combinations of the above search terms were used. There was no limit to the use of the word term “peripheral” to avoid omissions as much as possible. Only studies that were published in the English or Chinese language were considered.
This study conformed to the PRISMA guidelines (the Preferred Reporting Items for System Reviews and Meta-Analysis) statement.
Study selection
Our primary research question concerned the role of DM in the development of CIPN. Therefore, we looked for longitudinal studies that contained both exposure factors (DM) and clinical outcomes (incidence of CIPN) and comprised both diabetic and nondiabetic patients with or without CIPN. Case–control studies and cohort studies were included. The titles, abstracts, and subsequent full text of the retrieved publications were screened by two independent reviewers. Any disagreement between the reviewers was resolved by a third independent reviewer. Inclusion criteria were original case–control or cohort studies with outcome indicators that included the incidence of CIPN in diabetic or nondiabetic patients. Reviews, conference abstracts, case reports, editorials, letters to editors, repetitive publications, and studies for which data were unavailable were excluded. Endnote (V9.3.3, Clarivate Analytics) was used for literature screening and management. References in the literature that met the inclusion criteria were manually screened to prevent omissions.
Data extraction
Two independent reviewers extracted the following data from studies that met the inclusion criteria after duplicate checking: first author, time of publication, type of research, study population, grade of CIPN, antineoplastic drugs studied, outcome measure, and type of cancer.
Quality assessment
The Newcastle–Ottawa scale (NOS) was used to evaluate the quality of the included literature. Studies were judged on three aspects: selection, comparability, and exposure; under each aspect, there were several items for researchers to score, with a total maximum score of nine. Except for comparability (two points), the highest score for each of the other items was one point. Scores ranging from zero to three, four to six, and seven to nine represented low-, medium-, and high-quality studies, respectively. This scale is widely used in the quality assessment of nonrandomized studies and is suitable for use in case–control and cohort studies. The quality of the included studies was evaluated by two independent reviewers. A second review of the studies for which there was disagreement was conducted by the third reviewer. The quality of the studies was assessed using an adjusted NOS scale. Studies with scores greater than five were included in the subsequent meta-analyses. The details are described in Tables S1 and S2.
Statistical analysis
A meta-analysis of the data extracted from the studies that met the quality assessment was performed using Stata V15.1. Because the outcome indicator (incidence of CIPN) for this study was a bicategorical variable, odds ratios (ORs) and 95% confidence intervals (CIs) were used as the combined effect measures. Heterogeneity among studies was assessed using the chi-squared and I-squared (I2) tests. Random-effects models were used for pooling the results of different studies when P < 0.1 or I2 > 50%; otherwise, fixed-effects models were used for combined data. The risk of publication bias was calculated using Egger’s test and significant publication bias was determined at P < 0.05. Subgroup analysis was also conducted for important variables. The following potential influential stratified factors were considered: study type, study quality, evaluation instrument, and type of antineoplastic drug (mainly oxaliplatin and taxane). Interaction and heterogeneity tests were performed to detect the influence of each stratified factor on the relationship between DM and CIPN. In addition, a sensitivity analysis was constructed in which one study at a time was excluded and others were analyzed to estimate whether the results would be significantly affected by certain studies.
Results
Literature selection
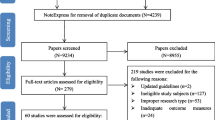
The search identified 417 publications derived from Chinese databases (Wanfang, CQVIP, and CNKI), and 2262 derived from PubMed, Embase, and WOS. Of these, 2187 were unique. Based on the titles and abstracts of all the articles screened by the reviewers, 2131 articles were excluded because they did not meet the inclusion criteria. After screening the full text of the 57 remaining studies that met the inclusion criteria, 25 studies were finally included in the meta-analysis as they clearly reported the incidence or severity of CIPN. Figure 1 shows the PRISMA flow chart of study identification and selection.
Quality assessment
The quality of the articles was independently assessed by at least two reviewers. All the 25 articles scored 5 points or more on the NOS scale, indicating a moderate to good overall study quality; 22 of the 25 studies were of high quality (scored 7 to 9 points). One study [15] reported the incidence of CIPN under two treatment regimens. Therefore, a total of 26 studies were included in the meta-analysis, including 9 cohort studies and 17 case–control studies. Nine of the 22 studies [15,16,17,18,19,20,21,22,23] reported a positive association between diabetes and CIPN, whereas the others reported no association. Table 1 shows the characteristics of the included studies. The quality assessment results of all the studies are shown in Tables S3 and S4.
Correlation between DM and CIPN
All 26 studies explored the association between CIPN and DM. Figure 2 shows the forest plot of these studies in the meta-analysis (a total of 8923 patients). Because the heterogeneity test showed that there was no statistical heterogeneity among the studies (I2 = 20.8%, P = 0.172), a fixed-effects model was used to analyze the outcome indicators. The results showed that there was a statistically significant positive correlation between DM and CIPN (OR = 1.60, 95% CI = 1.38–1.85, P < 0.01). Egger’s test (P = 0.824) showed no evidence of publication bias.
Subgroup and sensitivity analysis
Table 2 shows the subgroup analyses for study type, study quality, evaluation instrument, and type of antineoplastic drug. A positive association between DM and CIPN was observed in all subgroup analyses. Low or moderate of heterogeneity was identified within any subgroup. Notably, in the antineoplastic drug type subgroup analysis, eight studies reported oxaliplatin-induced neuropathy, while eight reported taxane-induced neuropathy. The results showed that the risk of CIPN in diabetic cancer patients treated with taxane-based drugs (OR = 1.47, 95% CI = 1.11–1.93) was higher than that in nondiabetic cancer patients. The same result could also be observed in the oxaliplatin subgroup (OR = 1.19, 95% CI = 0.86–1.65). The association between DM and CIPN risk was not significantly modified by antineoplastic drugs (P for interaction = 0.81). The combined ORs of overall risk estimates were consistent and without apparent fluctuation based on the sensitivity analyses, with a range from 1.53 (95% CI = 1.31–1.78) to 1.68 (95% CI = 1.44–1.95).
Discussion
Chemotherapy is an important treatment method for malignant tumors. Some antineoplastic drugs are irreplaceable for patients receiving postoperative adjuvant therapy, such as oxaliplatin for colorectal cancer and taxanes for breast cancer. However, many patients will develop peripheral neuropathy when administered these kinds of drugs, which affects quality of life and treatment compliance [6, 40]. No effective agents are currently recommended for the prevention of CIPN, while duloxetine has shown limited efficacy in clinical studies [41]. Consequently, preventing and treating CIPN remains clinically challenging. The identification of risk factors for CIPN enables clinicians to assess patients more accurately and pay more attention to it, whether in applying antineoplastic drugs or combining other therapies.
Neuropathy is also a common complication of DM, with up to 50% of diabetic patients developing peripheral neuropathy with disease progression [39]. Many cancer patients have a history of DM, and determining whether DM is a risk factor for CIPN is of importance in clinical practice. Our meta-analysis suggested that there was a positive correlation between DM and CIPN and the association was neither significantly modified by study quality, evaluation instrument, or type of antineoplastic drug nor substantially affected by any single study based on the results from our subgroup and sensitivity analyses.
A significant, robust, and positive association was found between DM and CIPN in all subgroups, except in the oxaliplatin subgroup. Although the mechanisms that lead to CIPN may differ between taxane and platinum, the nonsignificant association in oxaliplatin subgroup was likely due to fewer relevant studies and smaller number of sample size (n = 284) and, hence, insufficient statistical power. It is still important to note that different chemotherapeutic drugs affect distinct components of the nervous system. Both oxaliplatin and paclitaxel, as well as other agents, can cause DRG, axonal, and axonal component damage; however, paclitaxel and vincristine can also affect distal nerve terminals [42, 43]. Specifically, oxaliplatin and paclitaxel can cause mitochondrial dysfunction and oxidative stress injury in peripheral nerves. Paclitaxel can block axonal transport by inhibiting tubulin hydrolysis and interfering with normal axonal microtubule dynamics, while oxaliplatin can cause the abnormal function of ion channels, such as voltage-gated sodium channels, voltage-gated potassium channels, voltage-gated calcium channels, and transient receptor potential channels [9, 44, 45]. In general, paclitaxel- and other drug-induced peripheral neuropathies are similar to DPN in terms of mechanism and symptoms. Furthermore, most of the studies did not assess whether patients had DPN, especially mild symptoms, and did not record the duration of the DM. The assessment of DPN is different from that of CIPN, and the occurrence of DPN is closely related to the duration of this disease [39]. Consequently, it is impossible to know how many diabetic patients in these studies developed DPN, symptomatic, or otherwise. Previous study has reported that there was an association only in patients with complications of DM (i.e., PN from DM) and not in patients without complications [23]. Our meta-analysis preferred to show a significant association between DM and CIPN, regardless of the presence of comorbidities. However, such a conclusion may need to be further supported by additional studies on multivariables of DPN.
Given that DM is a high-risk factor for CIPN, a more detailed evaluation should be undertaken for cancer patients with diabetes when they are treated with chemotherapeutic agents that may cause peripheral neuropathy. First, the presence of diabetes and the duration of diabetes, especially the presence of DPN, should be fully evaluated before chemotherapy. If DPN is present, more intense monitoring for CIPN should be performed when using potentially neurotoxic drugs, and these patients could be recommended for clinical trials of CIPN prevention therapies. Second, when patients use drugs that can affect blood glucose, such as dexamethasone and glucose, attempts should be made to maintain blood glucose levels within the normal range during chemotherapy to reduce the possibility of the occurrence of CIPN and DPN.
This review had some limitations. First, although the quality assessment showed that most of the studies were of high quality, some studies nevertheless had a mild sample size, leading to potential bias. Second, most of the eligible studies were retrospective, and confounding factors may have biased the results. Third, there was no standardized definition of the incidence and grade of CIPN as the primary study outcome.
Conclusions
Our findings have important clinical implications; CIPN remains a common side effect of chemotherapy. Controversies continue regarding the effects of DM and CIPN risk. We conducted a meta-analysis of these controversial studies, enhancing the ability to detect associations and providing more reliable estimates. Taken together, this study provides evidence of a significant positive association between DM and risk of CIPN. Furthermore, a more detailed evaluation is warranted for cancer patients with diabetes when they are treated with antineoplastic drugs that have the potential to cause peripheral neuropathy.
Data and materials availability
The data sets supporting the results of this article are included within the article and its additional files.
References
Park SB, Goldstein D, Krishnan AV, Lin CS, Friedlander ML, Cassidy J, Koltzenburg M, Kiernan MC (2013) Chemotherapy-induced peripheral neurotoxicity: a critical analysis. CA Cancer J Clin 63:419–437. https://doi.org/10.3322/caac.21204
Wolf S, Barton D, Kottschade L, Grothey A, Loprinzi C (2008) Chemotherapy-induced peripheral neuropathy: prevention and treatment strategies. Eur J Cancer 44:1507–1515. https://doi.org/10.1016/j.ejca.2008.04.018
Boyette-Davis JA, Walters ET, Dougherty PM (2015) Mechanisms involved in the development of chemotherapy-induced neuropathy. Pain Manag 5:285–296. https://doi.org/10.2217/pmt.15.19
Ewertz M, Qvortrup C, Eckhoff L (2015) Chemotherapy-induced peripheral neuropathy in patients treated with taxanes and platinum derivatives. Acta Oncol 54:587–591. https://doi.org/10.3109/0284186X.2014.995775
Pachman DR, Qin R, Seisler DK, Smith EM, Beutler AS, Ta LE, Lafky JM, Wagner-Johnston ND, Ruddy KJ, Dakhil S, Staff NP, Grothey A, Loprinzi CL (2015) Clinical course of oxaliplatin-induced neuropathy: results from the randomized phase III trial N08CB (Alliance). J Clin Oncol 33(30):3416–3422. https://doi.org/10.1200/JCO.2014.58.8533
Mols F, Beijers T, Lemmens V, van den Hurk CJ, Vreugdenhil G, van de Poll-Franse LV (2013) Chemotherapy-induced neuropathy and its association with quality of life among 2- to 11-year colorectal cancer survivors: results from the population-based PROFILES registry. J Clin Oncol 31(21):2699–2707. https://doi.org/10.1200/JCO.2013.49.1514
Miltenburg NC, Boogerd W (2014) Chemotherapy-induced neuropathy: a comprehensive survey. Cancer Treat Rev 40:872–882. https://doi.org/10.1016/j.ctrv.2014.04.004
Hu S, Huang KM, Adams EJ, Loprinzi CL, Lustberg MB (2019) Recent developments of novel pharmacologic therapeutics for prevention of chemotherapy-induced peripheral neuropathy. Clin Cancer Res 25:6295–6301. https://doi.org/10.1158/1078-0432.CCR-18-2152
Sałat K (2020) Chemotherapy-induced peripheral neuropathy: part 1—current state of knowledge and perspectives for pharmacotherapy. Pharmac Rep 72(3):486–507. https://doi.org/10.1007/s43440-020-00109-y
Seretny M, Currie GL, Sena ES, Ramnarine S, Grant R, MacLeod MR, Colvin LA, Fallon M (2014) Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: a systematic review and meta-analysis. Pain 155(12):2461–2470. https://doi.org/10.1016/j.pain.2014.09.020
Kandula T, Park SB, Cohn RJ, Krishnan AV, Farrar MA (2016) Pediatric chemotherapy induced peripheral neuropathy: a systematic review of current knowledge. Cancer Treat Rev 50:118–128. https://doi.org/10.1016/j.ctrv.2016.09.005
Cliff J, Jorgensen AL, Lord R, Azam F, Cossar L, Carr DF, Pirmohamed M (2017) The molecular genetics of chemotherapy-induced peripheral neuropathy: a systematic review and meta-analysis. Crit Rev Oncol Hematol 120:127–140. https://doi.org/10.1016/j.critrevonc.2017.09.009
Timmins HC, Mizrahi D, Li T, Kiernan MC, Goldstein D, Park SB (2021) Metabolic and lifestyle risk factors for chemotherapy-induced peripheral neuropathy in taxane and platinum-treated patients: a systematic review. J Cancer Surviv. https://doi.org/10.1007/s11764-021-00988-x
Verstappen CC, Heimans JJ, Hoekman K, Postma TJ (2003) Neurotoxic complications of chemotherapy in patients with cancer: clinical signs and optimal management. Drugs 63:1549–1563. https://doi.org/10.2165/00003495-200363150-00003
Kus T, Aktas G, Kalender ME, Sevinc A, Kul S, Suner A, Ulker E, Camci C (2016) Taxane-induced peripheral sensorial neuropathy in cancer patients is associated with duration of diabetes mellitus: a single-center retrospective study. Support Care Cancer 24:1175–1179. https://doi.org/10.1007/s00520-015-2898-z
Chen C, Ding Q (2019) Effect of oxaliplatin on neurotoxicity of gastrointestinal tumors with diabetes mellitus. Diabetes New World 22(05):101–104
Gaballah A, Shafik A, Elhusseiny K, Ashraf M (2018) Chemotherapy-induced peripheral neuropathy in Egyptian patients: single institution retrospective analysis. Asian Pac J Cancer Prev 19:2223–2227. https://doi.org/10.22034/APJCP.2018.19.8.2223
Wang YQ, Yang XJ, Wei XC (2017) Analysis of factors affecting chemotherapy induced peripheral neurotoxicity. Tianjin Pharmacy 29(02):25–29
de la Morena BP, Conesa MA, Gonzalez-Billalabeitia E, Urrego E, Garcia-Garre E, Garcia-Martinez E, Poves MZ, Vicente V, de la Pena FA (2015) Delayed recovery and increased severity of paclitaxel-induced peripheral neuropathy in patients with diabetes. J Natl Compr Canc Netw 13:417–423. https://doi.org/10.6004/jnccn.2015.0057
Ding XF (2015) Exploring the effect of diabetes on paclitaxel nerve damage. Diabetes New World 18(19):37–38
Johnson C, Pankratz VS, Velazquez AI, Aakre JA, Loprinzi CL, Staff NP, Windebank AJ, Yang P (2015) Candidate pathway-based genetic association study of platinum and platinum-taxane related toxicity in a cohort of primary lung cancer patients. J Neurol Sci 349:124–128. https://doi.org/10.1016/j.jns.2014.12.041
Xue YJ, Gu LP, Xue TG, Bian CF, Wang H, Cui LM (2013) Clinical study of paclitaxel-induced neurotoxicity in tumor patients with/without diabetes mellitus. J Hebei Med Univ 34(11):1440–1442
Hershman DL, Till C, Wright JD, Awad D, Ramsey SD, Barlow WE, Minasian LM, Unger J (2016) Comorbidities and risk of chemotherapy-induced peripheral neuropathy among participants 65 years or older in Southwest Oncology Group Clinical Trials. J Clin Oncol 34(25):3014–3022. https://doi.org/10.1200/JCO.2015.66.2346
Molassiotis A, Cheng HL, Leung KT, Li YC, Wong KH, Au JSK, Sundar R, Chan A, Ng TR, Suen LKP, Chan CW, Yorke J, Lopez V (2019) Risk factors for chemotherapy-induced peripheral neuropathy in patients receiving taxane- and platinum-based chemotherapy. Brain Behav 9:e01312. https://doi.org/10.1002/brb3.1312
Hertz DL, Kidwell KM, Vangipuram K, Li F, Pai MP, Burness M, Griggs JJ, Schott AF, Van Poznak C, Hayes DF, Lavoie Smith EM, Henry NL (2018) Paclitaxel plasma concentration after the first infusion predicts treatment-limiting peripheral neuropathy. Clin Cancer Res 24:3602–3610. https://doi.org/10.1158/1078-0432.CCR-18-0656
Yamaguchi K, Kusaba H, Makiyama A, Mitsugi K, Uchino K, Tamura S, Shibata Y, Esaki T, Ito M, Takayoshi K, Tsuchihashi K, Arita S, Ariyama H, Akashi K, Baba E (2018) The risk factors for oxaliplatin-induced peripheral sensory neuropathy and thrombocytopenia in advanced gastric cancer. Cancer Chemother Pharmacol 82:625–633. https://doi.org/10.1007/s00280-018-3652-2
Dolan ME, El Charif O, Wheeler HE, Gamazon ER, Ardeshir-Rouhani-Fard S, Monahan P, Feldman DR, Hamilton RJ, Vaughn DJ, Beard CJ, Fung C, Kim J, Fossa SD, Hertz DL, Mushiroda T, Kubo M, Einhorn LH, Cox NJ, Travis LB (2017) clinical and genome-wide analysis of cisplatin-induced peripheral neuropathy in survivors of adult-onset cancer. Clin Cancer Res 23(19):5757–5768. https://doi.org/10.1158/1078-0432.CCR-16-3224
Song SJ, Min J, Suh SY, Jung SH, Hahn HJ, Im SA, Lee JY (2017) Incidence of taxane-induced peripheral neuropathy receiving treatment and prescription patterns in patients with breast cancer. Support Care Cancer 25:2241–2248. https://doi.org/10.1007/s00520-017-3631-x
Bao T, Basal C, Seluzicki C, Li SQ, Seidman AD, Mao JJ (2016) Long-term chemotherapy-induced peripheral neuropathy among breast cancer survivors: prevalence, risk factors, and fall risk. Breast Cancer Res Treat 159(2):327–333. https://doi.org/10.1007/s10549-016-3939-0
Tanishima H, Tominaga T, Kimura M, Maeda T, Shirai Y, Horiuchi T (2017) Hyperacute peripheral neuropathy is a predictor of oxaliplatin-induced persistent peripheral neuropathy. Support Care Cancer 25:1383–1389. https://doi.org/10.1007/s00520-016-3514-6
Pereira S, Fontes F, Sonin T, Dias T, Fragoso M, Castro-Lopes JM, Lunet N (2016) Chemotherapy-induced peripheral neuropathy after neoadjuvant or adjuvant treatment of breast cancer: a prospective cohort study. Support Care Cancer 24:1571–1581. https://doi.org/10.1007/s00520-015-2935-y
Shahriari-Ahmadi A, Fahimi A, Payandeh M, Sadeghi M (2015) Prevalence of oxaliplatin-induced chronic neuropathy and influencing factors in patients with colorectal cancer in Iran. Asian Pac J Cancer Prev 16:7603–7606. https://doi.org/10.7314/apjcp.2015.16.17.7603
Eckhoff L, Feddersen S, Knoop AS, Ewertz M, Bergmann TK (2015) Docetaxel-induced neuropathy: a pharmacogenetic case-control study of 150 women with early-stage breast cancer. Acta Oncol 54(4):535–542. https://doi.org/10.3109/0284186X.2014.969846
Wang XY, Wang ZW, Yuan C, Li HY (2013) Clinical predictive factor of oxaliplatin-induced peripheral neuropathy in colorectal cancer patients. Chin J Pract Med 40(20):71–73
Hashimoto N, Yokoyama K, Sadahira K, Ueda T, Tsukada Y, Okamoto S (2012) Itraconazole may increase the risk of early-onset bortezomib-induced peripheral neuropathy. Int J Hematol 96:758–763. https://doi.org/10.1007/s12185-012-1224-5
Kawakami K, Tunoda T, Takiguchi T, Shibata K, Ohtani T, Kizu J, Nishio M, Horai T, Hama T, Taguchi K (2012) Factors exacerbating peripheral neuropathy induced by paclitaxel plus carboplatin in non-small cell lung cancer. Oncol Res 20:179–185. https://doi.org/10.3727/096504012x13522227232192
Vincenzi B, Frezza AM, Schiavon G, Spoto C, Silvestris N, Addeo R, Catalano V, Graziano F, Santini D, Tonini G (2013) Identification of clinical predictive factors of oxaliplatin-induced chronic peripheral neuropathy in colorectal cancer patients treated with adjuvant Folfox IV. Support Care Cancer 21:1313–1319. https://doi.org/10.1007/s00520-012-1667-5
Uwah AN, Ackler J, Leighton JC Jr, Pomerantz S, Tester W (2012) The effect of diabetes on oxaliplatin-induced peripheral neuropathy. Clin Colorectal Cancer 11:275–279. https://doi.org/10.1016/j.clcc.2012.05.002
Ramanathan RK, Rothenberg ML, de Gramont A, Tournigand C, Goldberg RM, Gupta S, Andre T (2010) Incidence and evolution of oxaliplatin-induced peripheral sensory neuropathy in diabetic patients with colorectal cancer: a pooled analysis of three phase III studies. Ann Oncol 21:754–758. https://doi.org/10.1093/annonc/mdp509
Soveri LM, Lamminmaki A, Hanninen UA, Karhunen M, Bono P, Osterlund P (2019) Long-term neuropathy and quality of life in colorectal cancer patients treated with oxaliplatin containing adjuvant chemotherapy. Acta Oncol 58:398–406. https://doi.org/10.1080/0284186X.2018.1556804
Loprinzi CL, Lacchetti C, Bleeker J, Cavaletti G, Chauhan C, Hertz DL, Kelley MR, Lavino A, Lustberg MB, Paice JA, Schneider BP, Lavoie Smith EM, Smith ML, Smith TJ, Wagner-Johnston N, Hershman DL (2020) Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: ASCO Guideline Update. J Clin Oncol 38:3325–3348. https://doi.org/10.1200/JCO.20.01399
Melli G, Jack C, Lambrinos GL, Ringkamp M, Hoke A (2006) Erythropoietin protects sensory axons against paclitaxel-induced distal degeneration. Neurobiol Dis 24:525–530. https://doi.org/10.1016/j.nbd.2006.08.014
Avan A, Postma TJ, Ceresa C, Avan A, Cavaletti G, Giovannetti E, Peters GJ (2015) Platinum‐induced neurotoxicity and preventive strategies: past, present, and future. Oncologist 20(4):411–432. https://doi.org/10.1634/theoncologist.2014-0044
Areti A, Yerra VG, Naidu V, Kumar A (2014) Oxidative stress and nerve damage: role in chemotherapy induced peripheral neuropathy. Redox Biol 2:289–295. https://doi.org/10.1016/j.redox.2014.01.006
Trecarichi A, Flatters SJL (2019) Chapter Five - Mitochondrial dysfunction in the pathogenesis of chemotherapy-induced peripheral neuropathy. In: Fernyhough P, Calcutt NA (eds) International review of neurobiology. Academic Press, pp 83–126
Funding
This work was supported by the National Natural Science Foundation of China (no. 82004339), Project of National Clinical Research Base of Traditional Chinese Medicine in Jiangsu Province (nos. JD2019SZXYB02, JD2019SZXYB16), Scientific Research Project of Jiangsu Provincial Health Commission (no. H2019095), Jiangsu Science and Technology Department Social Development-Clinical Frontier Technology (nos. BE2019767, BRA2019100), and Jiangsu Province TCM Leading Talent Training Project (no. SLJ0211).
Author information
Authors and Affiliations
Contributions
Conceptualization: Hong Lu; methodology: Jialin Yu and Chen Chen; formal analysis and investigation: Zhancheng Gu; writing (original draft preparation): Jialin Gu; writing (review and editing): Guoli Wei; funding acquisition: Jiege Huo; resources: Miao Hu; supervision: Ling Liu.
Corresponding authors
Ethics declarations
Conflicts of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary 1
Table S1: Adjusted Newcastle–Ottawa scale (NOS) for the cohort studies (PDF 64 kb)
Supplementary 2
Table S2: Adjusted Newcastle–Ottawa scale (NOS) for the case-control studies (PDF 60 kb)
Supplementary 3
Table S3: Adjusted Newcastle–Ottawa scale (NOS) scores for the cohort studies (PDF 53 kb)
Supplementary 4
Table S4: Adjusted Newcastle–Ottawa scale (NOS) scores for the case-control studies (PDF 51 kb)
Supplementary 5
Table S5: PRISMA 2009 Checklist (PDF 95 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gu, J., Lu, H., Chen, C. et al. Diabetes mellitus as a risk factor for chemotherapy-induced peripheral neuropathy: a meta-analysis. Support Care Cancer 29, 7461–7469 (2021). https://doi.org/10.1007/s00520-021-06321-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-021-06321-7