Abstract
Purpose
Dexamethasone (DEX)-sparing strategies (one-day DEX) with palonosetron as doublet antiemetic prophylaxis have previously been studied. However, DEX-sparing regimens with 5-hydroxytryptamine-3 receptor antagonist (5-HT3RA) and aprepitant (APR), as triplet antiemetic prophylaxis, have not been evaluated. This study aimed to evaluate the efficacy of a combination of 5-HT3RA, APR, and DEX on day 1 of carboplatin (CBDCA)-based chemotherapy in patients with lung cancer.
Methods
Data were pooled from a nationwide, multicenter, prospective observational study using propensity score–matched analysis to compare the incidence of chemotherapy-induced nausea and vomiting (CINV) between one- and multiple-day DEX regimens in combination with 5-HT3RA plus APR.
Results
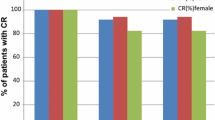
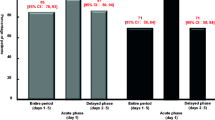
Incidence of delayed nausea was significantly higher in the one-day than in the multiple-day DEX group. Incidence of nausea was also significantly higher in the one-day than in the multiple-day DEX group on days 3–5. Kaplan–Meier curves for nausea showed a significant difference between the two groups; however, there was no significant difference in the occurrence of vomiting or the Kaplan–Meier curves of time to vomiting.
Conclusion
To the best of our knowledge, this study is the first to evaluate the efficacy of a DEX-sparing regimen by comparing one- and multiple-day DEX combined with 5-HT3RA and APR concerning CINV incidence in lung cancer patients receiving CBDCA-based chemotherapy. Antiemetic regimens of one-day DEX result in poor control of delayed nausea; therefore, we recommend the application of the DEX-sparing strategy only after careful patient selection while considering the development of nausea.





Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the study group. However, restrictions apply to the availability of these data, which were used under a license for the current study and are thus not publicly available. Data are, nevertheless, available from the authors upon reasonable request and with permission of the study group.
References
Roila F, Warr D, Hesketh PJ, Gralla R, Herrstedt J, Jordan K, Aapro M, Ballatori E, Rapoport B (2017) 2016 Updated MASCC/ESMO consensus recommendations: prevention of nausea and vomiting following moderately emetogenic chemotherapy. Support Care Cancer 25:289–294. https://doi.org/10.1007/s00520-016-3365-1
Hesketh PJ, Kris MG, Basch E, Bohlke K, Barbour SY, Clark-Snow RA, Danso MA, Dennis K, Dupuis LL, Dusetzina SB, Eng C, Feyer PC, Jordan K, Noonan K, Sparacio D, Lyman GH (2020) Antiemetics: ASCO Guideline update. J Clin Oncol 38:2782–2797. https://doi.org/10.1200/JCO.20.01296
NCCN clinical practice guidelines in oncology (2020) Antiemetics. version 2, vol. 2020. https://www.nccn.org/professionals/physician_gls/pdf/antiemesis.pdf. Accessed 20 Oct 2020
Aogi K, Takeuchi H, Saeki T, Aiba K, Tamura K, Iino K, Imamura CK, Okita K, Kagami Y, Tanaka R, Nakagawa K, Fujii H, Boku N, Wada M, Akechi T, Iihara H, Ohtani S, Okuyama A, Ozawa K, Kim YI, Sasaki H, Shima Y, Takeda M, Nagasaki E, Nishidate T, Higashi T, Hirata K (2020) Optimizing antiemetic treatment for chemotherapy-induced nausea and vomiting in Japan: update summary of the 2015 Japan Society of Clinical Oncology Clinical Practice Guidelines for Antiemesis. Int J Clin Oncol published online 08 November 2020. https://doi.org/10.1007/s10147-020-01818-3
Iihara H, Shimokawa M, Hayashi T, Kawazoe H, Saeki T, Aiba K, Tamura K (2020) A nationwide, multicenter registry study of antiemesis for carboplatin-based chemotherapy-induced nausea and vomiting in Japan. Oncologist 25:e373–e380. https://doi.org/10.1634/theoncologist.2019-0292
Shimokawa M, Hayashi T, Kogawa T, Matsui R, Mizuno M, Kikkawa F, Saeki T, Aiba K, Tamura K (2019) Evaluation of combination antiemetic therapy on CINV in patients with gynecologic cancer receiving TC chemotherapy. Anticancer Res 39:225–230. https://doi.org/10.21873/anticanres.13101
Ito Y, Karayama M, Inui N, Kuroishi S, Nakano H, Nakamura Y, Yokomura K, Toyoshima M, Shirai T, Masuda M, Yamada T, Yasuda K, Hayakawa H, Suda T, Chida K (2014) Aprepitant in patients with advanced non-small-cell lung cancer receiving carboplatin-based chemotherapy. Lung Cancer 84:259–264. https://doi.org/10.1016/j.lungcan.2014.03.017
Karayama M, Inui N, Tanaka K, Yasui H, Hozumi H, Suzuki Y, Furuhashi K, Fujisawa T, Enomoto N, Nakamura Y, Suda T (2018) Prophylactic aprepitant is better than salvage for carboplatin-based chemotherapy: a propensity score-matched analysis. Med Oncol 35:139. https://doi.org/10.1007/s12032-018-1199-z
Jordan K, Blättermann L, Hinke A, Müller-Tidow C, Jahn F (2018) Is the addition of a neurokinin-1 receptor antagonist beneficial in moderately emetogenic chemotherapy?-a systematic review and meta-analysis. Support Care Cancer 26:21–32. https://doi.org/10.1007/s00520-017-3857-7
Barbour SY (2012) Corticosteroids in the treatment of chemotherapy-induced nausea and vomiting. J Natl Compr Canc Netw 10:493–499. https://doi.org/10.6004/jnccn.2012.0049
Vardy J, Chiew KS, Galica J, Pond GR, Tannock IF (2006) Side effects associated with the use of dexamethasone for prophylaxis of delayed emesis after moderately emetogenic chemotherapy. Br J Cancer 94:1011–1015. https://doi.org/10.1038/sj.bjc.6603048
Stoltz R, Cyong JC, Shah A, Parisi S (2004) Pharmacokinetic and safety evaluation of palonosetron, a 5-hydroxytryptamine-3 receptor antagonist, in U.S. and Japanese healthy subjects. J Clin Pharmacol 44:520–531. https://doi.org/10.1177/0091270004264641
Rojas C, Thomas AG, Alt J, Stathis M, Zhang J, Rubenstein EB, Sebastiani S, Cantoreggi S, Slusher BS (2010) Palonosetron triggers 5-HT3 receptor internalization and causes prolonged inhibition of receptor function. Eur J Pharmacol 626:193–199. https://doi.org/10.1016/j.ejphar.2009.10.002
Aapro M, Fabi A, Nolè F, Medici M, Steger G, Bachmann C, Roncoroni S, Roila F (2010) Double-blind, randomised, controlled study of the efficacy and tolerability of palonosetron plus dexamethasone for 1 day with or without dexamethasone on days 2 and 3 in the prevention of nausea and vomiting induced by moderately emetogenic chemotherapy. Ann Oncol 21:1083–1088. https://doi.org/10.1093/annonc/mdp584
Komatsu Y, Okita K, Yuki S, Furuhata T, Fukushima H, Masuko H, Kawamoto Y, Isobe H, Miyagishima T, Sasaki K, Nakamura M, Ohsaki Y, Nakajima J, Tateyama M, Eto K, Minami S, Yokoyama R, Iwanaga I, Shibuya H, Kudo M, Oba K, Takahashi Y (2015) Open-label, randomized, comparative, phase III study on effects of reducing steroid use in combination with palonosetron. Cancer Sci 106:891–895. https://doi.org/10.1111/cas.12675
Celio L, Frustaci S, Denaro A, Buonadonna A, Ardizzoia A, Piazza E, Fabi A, Capobianco AM, Isa L, Cavanna L, Bertolini A, Bichisao E, Bajetta E (2011) Palonosetron in combination with 1-day versus 3-day dexamethasone for prevention of nausea and vomiting following moderately emetogenic chemotherapy: a randomized, multicenter, phase III trial. Support Care Cancer 19:1217–1225. https://doi.org/10.1007/s00520-010-0941-7
Furukawa N, Kanayama S, Tanase Y, Ito F (2015) Palonosetron in combination with 1-day versus 3-day dexamethasone to prevent nausea and vomiting in patients receiving paclitaxel and carboplatin. Support Care Cancer 23:3317–3322. https://doi.org/10.1007/s00520-015-2760-3
Kosaka Y, Tanino H, Sengoku N, Minatani N, Kikuchi M, Nishimiya H, Waraya M, Katoh H, Enomoto T, Sato T, Kuranami M, Watanabe M (2016) Phase II randomized, controlled trial of 1 day versus 3 days of dexamethasone combined with palonosetron and aprepitant to prevent nausea and vomiting in Japanese breast cancer patients receiving anthracycline-based chemotherapy. Support Care Cancer 24:1405–1411. https://doi.org/10.1007/s00520-015-2905-4
Okada Y, Oba K, Furukawa N, Kosaka Y, Okita K, Yuki S, Komatsu Y, Celio L, Aapro M (2019) One-day versus three-day dexamethasone in combination with palonosetron for the prevention of chemotherapy-induced nausea and vomiting: a systematic review and individual patient data-based meta-analysis. Oncologist 24:1593–1600. https://doi.org/10.1634/theoncologist.2019-0133
Celio L, Bonizzoni E, Zattarin E, Codega P, de Braud F, Aapro M (2019) Impact of dexamethasone-sparing regimens on delayed nausea caused by moderately or highly emetogenic chemotherapy: a meta-analysis of randomised evidence. BMC Cancer 19:1268. https://doi.org/10.1186/s12885-019-6454-y
Ito Y, Tsuda T, Minatogawa H, Kano S, Sakamaki K, Ando M, Tsugawa K, Kojima Y, Furuya N, Matsuzaki K, Fukuda M, Sugae S, Ohta I, Arioka H, Tokuda Y, Narui K, Tsuboya A, Suda T, Morita S, Boku N, Yamanaka T, Nakajima TE (2018) Placebo-controlled, double-blinded phase III study comparing dexamethasone on day 1 with dexamethasone on days 1 to 3 with combined neurokinin-1 receptor antagonist and palonosetron in high-emetogenic chemotherapy. J Clin Oncol 36:1000–1006. https://doi.org/10.1200/JCO.2017.74.4375
Tamura K, Aiba K, Saeki T, Nakanishi Y, Kamura T, Baba H, Yoshida K, Yamamoto N, Kitagawa Y, Maehara Y, Shimokawa M, Hirata K, Kitajima M, CINV Study group of Japan (2015) Testing the effectiveness of antiemetic guidelines: results of a prospective registry by the CINV Study Group of Japan. Int J Clin Oncol 20:855–865. https://doi.org/10.1007/s10147-015-0786-7
Italian Group for Antiemetic, Research (1995) Dexamethasone, granisetron, or both for the prevention of nausea and vomiting during chemotherapy for cancer. N Engl J Med 1:1–5. https://doi.org/10.1056/NEJM199501053320101
Hayashi T, Shimokawa M, Matsuo K, Miyoshi T, Toriyama Y, Yokota C, Taniguchi J, Hanada K, Tsumagari K, Okubo N, Koutake Y, Sakata K, Kawamata Y, Goto T, Tsurusaki Y, Koyabu M (2018) Risk factors for delayed chemotherapy-induced nausea and vomiting with low-emetic-risk chemotherapy: a prospective, observational, multicenter study. Cancer Manag Res 10:4249–4255. https://doi.org/10.2147/cmar.S176574
Navari RM, Qin R, Ruddy KJ, Liu H, Powell SF, Bajaj M, Dietrich L, Biggs D, Lafky JM, Loprinzi CL (2016) Olanzapine for the prevention of chemotherapy-induced nausea and vomiting. N Engl J Med 375:134–142. https://doi.org/10.1056/NEJMoa1515725
Hashimoto H, Abe M, Tokuyama O, Mizutani H, Uchitomi Y, Yamaguchi T, Hoshina Y, Sakata Y, Takahashi TY, Nakashima K, Nakao M, Takei D, Zenda S, Mizukami K, Iwasa S, Sakurai M, Yamamoto N, Ohe Y (2020) Olanzapine 5 mg plus standard antiemetic therapy for the prevention of chemotherapy-induced nausea and vomiting (J-FORCE): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 21:242–249. https://doi.org/10.1016/S1470-2045(19)30678-3
Tanaka K, Inui N, Karayama M, Yasui H, Hozumi H, Suzuki Y, Furuhashi K, Fujisawa T, Enomoto N, Nakamura Y, Kusagaya H, Matsuura S, Uto T, Hashimoto D, Matsui T, Asada K, Suda T (2019) Olanzapine-containing antiemetic therapy for the prevention of carboplatin-induced nausea and vomiting. Cancer Chemother Pharmacol 84:147–153. https://doi.org/10.1007/s00280-019-03868-5
Navari RM, Nagy CK, Gary SE (2013) The use of olanzapine versus metoclopramide for the treatment of breakthrough chemotherapy-induced nausea and vomiting in patients receiving highly emetogenic chemotherapy. Support Care Cancer 21:1655–1663. https://doi.org/10.1007/s00520-012-1710-6
Nakamura M, Ishiguro A, Muranaka T, Fukushima H, Yuki S, Ono K, Murai T, Matsuda C, Oba A, Itaya K, Sone T, Yagisawa M, Koike Y, Endo A, Tsukuda Y, Ono Y, Kudo T, Nagasaka A, Nishikawa S, Komatsu Y (2017) A prospective observational study on effect of short-term periodic steroid premedication on bone metabolism in gastrointestinal cancer (ESPRESSO-01). Oncologist 22:592–600. https://doi.org/10.1634/theoncologist.2016-0308
Acknowledgements
We wish to thank Dr. Kazuo Tamura for providing support as the principal investigator of the nationwide, multicenter, observational study that collected the data used in this study. We also thank all of the participants of the aforementioned study and their families.
Code availability
N/A
Author information
Authors and Affiliations
Contributions
TH conceived the study concept. TH, MS, and FM conducted the claim data analysis. MS performed the statistical analyses. KM and FM provided technical support. TH, MS, KM, FM, KK, TN, and TE contributed to the interpretation of data and assisted in the preparation of the manuscript. TH and MS prepared the initial draft of the manuscript. TH, MS, FM, KM, KK, TN, and TE conducted the critical revision of the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Consent to participate
All participants provided written informed consent.
Consent for publication
N/A
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 17 kb)
Rights and permissions
About this article
Cite this article
Hayashi, T., Shimokawa, M., Mizuki, F. et al. Efficacy of one-day versus multiple-day dexamethasone for chemotherapy-induced nausea and vomiting in lung cancer patients receiving carboplatin-based chemotherapy: a propensity score–matched analysis. Support Care Cancer 29, 5029–5035 (2021). https://doi.org/10.1007/s00520-021-06061-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-021-06061-8