Abstract
Purpose
The aim of the present study was to evaluate the efficacy and toxicity of palonosetron (PAL) and dexamethasone (DEX) on day 1 only in patients with gynecologic cancer receiving paclitaxel combined with carboplatin (TC). The primary endpoint was to evaluate the complete response (CR) rate in the delayed phase.
Methods
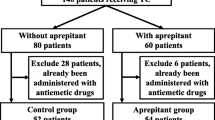
This study was a randomized phase 2. Regardless of assignment to either study arm, all patients received an intravenous prophylactic regimen of DEX (20 mg) within 15 min and then an intravenous dose of PAL (0.75 mg) as a bolus given 30 min before initiation of TC on day 1. Patients in the DEX 1-day group received no additional DEX on days 2 and 3. Patients in the DEX 3-day group received DEX (8 mg) orally on days 2 and 3.
Results
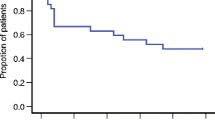
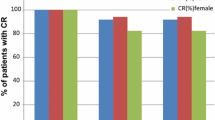
Eighty-two patients had evaluable data on the primary outcome. The CR rates in the delayed phase between the two groups were not statistically significantly different (3-day group, 76.9 % [30/39]; 1-day group 69.8 % [30/43]; p = 0.4652). The frequency of constipation and insomnia which were antiemetic treatment-related adverse events was similar between two groups, and no serious adverse events occurred.
Conclusions
Administration of a combination of PAL and DEX 1 day may prevent chemotherapy-induced nausea and vomiting (CINV) in the delayed phase for TC as well as administration of DEX 3 days. Further evaluation of the antiemetic regimen of combination of PAL and DEX 1 day for TC is warranted in future phase 3 trials.

Similar content being viewed by others
References
Basch E, Prestrud AA, Hesketh PJ, et al (2011) Antiemetics: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol 29:4189–98
Roila F, Herrstedt J, Aapro M et al (2010) Guideline update for MASCC and ESMO in the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting: results of the Perugia consensus conference. Ann Oncol 21(Suppl 5):v232–43
NCCN Clinical Practice Guideline in Oncology. Antiemesis. http://wwwnccnorg/professionals/physician_gls/pdf/antiemesispdf 2014; Accessed 5 October 2012.
Wong EH, Clark R, Leung E et al (1995) The interaction of RS 25259-197, a potent and selective antagonist, with 5-HT3 receptors, in vitro. Br J Pharmacol 114:851–9
Stoltz R, Cyong JC, Shah A, Parisi S (2004) Pharmacokinetic and safety evaluation of palonosetron, a 5-hydroxytryptamine-3 receptor antagonist, in U.S. and Japanese healthy subjects. J Clin Pharmacol 44:520–31
Vardy J, Chiew KS, Galica J, Pond GR, Tannock IF (2006) Side effects associated with the use of dexamethasone for prophylaxis of delayed emesis after moderately emetogenic chemotherapy. Br J Cancer 94:1011–5
Aapro M, Fabi A, Nole F et al (2010) Double-blind, randomised, controlled study of the efficacy and tolerability of palonosetron plus dexamethasone for 1 day with or without dexamethasone on days 2 and 3 in the prevention of nausea and vomiting induced by moderately emetogenic chemotherapy. Ann Oncol 21:1083–8
Celio L, Frustaci S, Denaro A et al (2011) Palonosetron in combination with 1-day versus 3-day dexamethasone for prevention of nausea and vomiting following moderately emetogenic chemotherapy: a randomized, multicenter, phase III trial. Support Care Cancer 19:1217–25
Perwitasari DA, Atthobari J, Mustofa M et al (2012) Impact of chemotherapy-induced nausea and vomiting on quality of life in Indonesian patients with gynecologic cancer. Int J Gynecol Cancer 22:139–45
Damian S, Celio L, De Benedictis E et al (2013) Is a dexamethasone-sparing strategy capable of preventing acute and delayed emesis caused by combined doxorubicin and paclitaxel for breast cancer? Analysis of a phase II trial. Oncology 84:371–7
du Bois A, Luck HJ, Meier W et al (2003) A randomized clinical trial of cisplatin/paclitaxel versus carboplatin/paclitaxel as first-line treatment of ovarian cancer. J Natl Cancer Inst 95:1320–9
Vasey PA, Jayson GC, Gordon A et al (2004) Phase III randomized trial of docetaxel-carboplatin versus paclitaxel-carboplatin as first-line chemotherapy for ovarian carcinoma. J Natl Cancer Inst 96:1682–91
Furukawa N, Akasaka J, Shigemitsu A et al (2014) Evaluation of the relation between patient characteristics and the state of chemotherapy-induced nausea and vomiting in patients with gynecologic cancer receiving paclitaxel and carboplatin. Arch Gynecol Obstet 289:859–64
Aapro MS, Grunberg SM, Manikhas GM et al (2006) A phase III, double-blind, randomized trial of palonosetron compared with ondansetron in preventing chemotherapy-induced nausea and vomiting following highly emetogenic chemotherapy. Ann Oncol 17:1441–9
Pollera CF, Giannarelli D (1989) Prognostic factors influencing cisplatin-induced emesis. Definition and validation of a predictive logistic model. Cancer 64:1117–22
Roila F, Boschetti E, Tonato M et al (1991) Predictive factors of delayed emesis in cisplatin-treated patients and antiemetic activity and tolerability of metoclopramide or dexamethasone. A randomized single-blind study. Am J Clin Oncol 14:238–42
du Bois A, Meerpohl HG, Vach W, Kommoss FG, Fenzl E, Pfleiderer A (1992) Course, patterns, and risk-factors for chemotherapy-induced emesis in cisplatin-pretreated patients: a study with ondansetron. Eur J Cancer 28:450–7
Hesketh PJ, Aapro M, Street JC, Carides AD (2010) Evaluation of risk factors predictive of nausea and vomiting with current standard-of-care antiemetic treatment: analysis of two phase III trials of aprepitant in patients receiving cisplatin-based chemotherapy. Support Care Cancer 18:1171–7
Esra YE, Aziz Y, Zeki U et al (2011) Is chemotherapy-induced nausea and vomiting lower in smokers? Int J Clin Pharmacol Ther 49:709–12
Sekine I, Segawa Y, Kubota K, Saeki T (2013) Risk factors of chemotherapy-induced nausea and vomiting: index for personalized antiemetic prophylaxis. Cancer Sci 104:711–7
Warr DG, Street JC, Carides AD (2011) Evaluation of risk factors predictive of nausea and vomiting with current standard-of-care antiemetic treatment: analysis of phase 3 trial of aprepitant in patients receiving adriamycin-cyclophosphamide-based chemotherapy. Support Care Cancer 19:807–13
Celio L, Bonizzoni E, Bajetta E, Sebastiani S, Perrone T, Aapro MS (2013) Palonosetron plus single-dose dexamethasone for the prevention of nausea and vomiting in women receiving anthracycline/cyclophosphamide-containing chemotherapy: meta-analysis of individual patient data examining the effect of age on outcome in two phase III trials. Support Care Cancer 21:565–73
Acknowledgment
The authors are grateful to Dr. Ryuji Kawaguchi and Dr. Shozo Yoshida for the continuing interest and helpful discussions.
Conflict of interest
The authors have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Furukawa, N., Kanayama, S., Tanase, Y. et al. Palonosetron in combination with 1-day versus 3-day dexamethasone to prevent nausea and vomiting in patients receiving paclitaxel and carboplatin. Support Care Cancer 23, 3317–3322 (2015). https://doi.org/10.1007/s00520-015-2760-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-015-2760-3