Abstract
Purpose
The Japan Adult Leukemia Study Group (JALSG) AML201 protocols are regimens for remission induction and consolidation chemotherapy of acute myeloid leukemia (AML) and have been widely accepted in Japan since 2001. Management of infectious complications during chemotherapy has a key role in the supportive care of AML patients.
Methods
By using case report forms collected in December 2001 and December 2005, we retrospectively analyzed the infectious complications in adult patients treated by using the JALSG AML201 protocols against AML (excluding promyelocytic leukemia).
Results
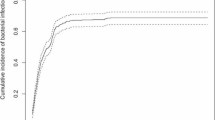
Of 980 patients, 80.2% experienced febrile neutropenia (FN), 8.3% bacteremia/fungemia, and 10.3% pulmonary infection at least once during remission-induction chemotherapy. Gram-positive bacteremia accounted for 65.1% of bacteremia/fungemia in 2001–2005, compared with 38.2% in 1987–1991 and 45.9% in 1992–1995. Of 750 patients, 81.9% experienced FN, 21.9% bacteremia/fungemia, and 9.1% pulmonary infection at least once during consolidation chemotherapy. During consolidation chemotherapy, bacteremia/fungemia and pulmonary infection were significantly more frequent in the high-dose cytarabine (HDAC) arm than in the conventional multiagent arm (25.9 vs. 17.9% and 12.7 vs. 7.7%, respectively). Invasive pulmonary aspergillosis accounted for 15.8% of pulmonary infections during remission induction and 19.7% during consolidation chemotherapy.
Conclusions
Our data suggest that patterns of infectious complications have changed between 1987 and 2005, possibly because of chemoprophylaxis with oral fluoroquinolones and improved diagnosis of invasive pulmonary aspergillosis by serum antigen analysis.


Similar content being viewed by others
References
Freifeld AG, Bow EJ, Sepkowitz KA, Boeckh MJ, Ito JI, Mullen CA, Raad II, Rolston KV, Young JA, Wingard JR, Infectious Diseases Society of America (2011) Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis 52:e56–e93. https://doi.org/10.1093/cid/cir073
Klastersky J, de Naurois J, Rolston K, Rapoport B, Maschmeyer G, Aapro M, Herrstedt J, ESMO Guidelines Committee (2016) Management of febrile neutropaenia: ESMO clinical practice guidelines. Ann Oncol 27:v111–v118. https://doi.org/10.1093/annonc/mdw325
Engels EA, Lau J, Barza M (1998) Efficacy of quinolone prophylaxis in neutropenic cancer patients: a meta-analysis. J Clin Oncol 16:1179–1187. https://doi.org/10.1200/JCO.1998.16.3.1179
Kobayashi T, Miyawaki S, Tanimoto M, Kuriyama K, Murakami H, Yoshida M, Minami S, Minato K, Tsubaki K, Ohmoto E, Oh H, Jinnai I, Sakamaki H, Hiraoka A, Kanamaru A, Takahashi I, Saito K, Naoe T, Yamada O, Asou N, Kageyama S, Emi N, Matsuoka A, Tomonaga M, Ohno R (2012) Randomized trials between behenoyl cytarabine and cytarabine in combination induction and consolidation therapy, and with or without ubenimex after maintenance/intensification therapy in adult acute myeloid leukemia. The Japan Leukemia Study Group. J Clin Oncol 14:204–213. https://doi.org/10.1200/JCO.1996.14.1.204
Ohno R, Kobayashi T, Tanimoto M, Hiraoka A, Imai K, Asou N, Tomonaga M, Tsubaki K, Takahashi I, Kodera Y, Yoshida M, Murakami H, Naoe T, Shimoyama M, Tsukada T, Takeo T, Teshima H, Onozawa Y, Fujimoto K, Kuriyama K, Horiuchi A, Kimura I, Minami S, Miura Y, Kageyama S, Tahara T, Masaoka T, Shirakawa S, Saito H (1993) Randomized study of individualized induction therapy with or without VCR, and of maintenance of 4 or 12 courses in adult AML: JALSG-AML87. Japan Adult Leukemia Study Group (JALSG). Cancer 71:3888–3895
Miyawaki S, Tanimoto M, Kobayashi T, Minami S, Tamura J, Omoto E, Kuriyama K, Hatake K, Saito K, Kanamaru A, Oh H, Ohtake S, Asou N, Sakamaki H, Yamada O, Jinnai I, Tsubaki K, Takeyama K, Hiraoka A, Matsuda S, Takahashi M, Shimazaki C, Adachi K, Kageyama S, Ohno R (1999) No beneficial effect from addition of etoposide to daunorubicin, cytarabine, and 6-mercaptopurine in individualized induction therapy of adult acute myeloid leukemia: the JALSG-AML92 study. Japan Adult Leukemia Study Group. Int J Hematol 70:97–104
Ohtake S, Miyawaki S, Kiyoi H, Miyazaki Y, Okumura H, Matsuda S, Nagai T, Kishimoto Y, Okada M, Takahashi M, Handa H, Takeuchi J, Kageyama S, Asou N, Yagasaki F, Maeda Y, Ohnishi K, Naoe T, Ohno R (2010) Randomized trial of response-oriented individualized versus fixed-schedule induction chemotherapy with idarubicin and cytarabine in adult acute myeloid leukemia: the JALSG AML95 study. Int J Hematol 91:276–283. https://doi.org/10.1007/s12185-009-0480-5
Miyawaki S, Sakamaki H, Ohtake S, Emi N, Yagasaki F, Mitani K, Matsuda S, Kishimoto Y, Miyazaki Y, Asou N, Matsushima T, Takahashi M, Ogawa Y, Honda S, Ohno R, Japan Adult Leukemia Study Group AML 97 Study (2005) A randomized, postremission comparison of four courses of standard-dose consolidation therapy without maintenance therapy versus three courses of standard-dose consolidation with maintenance therapy in adults with acute myeloid leukemia. Cancer 104:2726–2734. https://doi.org/10.1002/cncr.21493
Ohtake S, Miyawaki S, Fujita H, Kiyoi H, Shinagawa K, Usui N, Okumura H, Miyamura K, Nakaseko C, Miyazaki Y, Fujieda A, Nagai T, Yamane T, Taniwaki M, Takahashi M, Yagasaki F, Kimura Y, Asou N, Sakamaki H, Handa H, Honda S, Ohnishi K, Naoe T, Ohno R (2011) Randomized study of induction therapy comparing standard-dose idarubicin with high-dose daunorubicin in adult patients with previously untreated acute myeloid leukemia: the JALSG AML201 study. Blood 117:2358–2366. https://doi.org/10.1182/blood-2010-03-273243
Miyawaki S, Ohtake S, Fujisawa S et al (2011) A randomized comparison of 4 courses of standard-dose multiagent chemotherapy versus 3 courses of high-dose cytarabine alone in postremission therapy for acute myeloid leukemia in adults: the JALSG AML201 study. Blood 117:2366–2373. https://doi.org/10.1182/blood-2010-07-295279
Yoshida M, Ohno R (2004) Current antimicrobial usage for the management of infections in leukemic patients in Japan: results of a survey. Clin Infect Dis 39:S11–S14. https://doi.org/10.1086/383044
Fujita H, Yoshida M, Miura K, Sano T, Kito K, Takahashi M, Shigeno K, Kanda Y, Akiyama N, Hatsumi N, Ohnishi K, Miyawaki S, Naoe T (2009) Management of infection in patients with acute leukemia during chemotherapy in Japan: questionnaire analysis by the Japan adult leukemia study group. Int J Hematol 90:191–198. https://doi.org/10.1007/s12185-009-0367-5
Kimura S, Fujita H, Kato H et al (2017) Management of infection during chemotherapy for acute leukemia in Japan: a nationwide questionnaire-based survey by the Japan Adult Leukemia Study Group. Support Care Cancer 25:3515–3521. https://doi.org/10.1007/s00520-017-3775-8
Masaoka T (2004) Evidence-based recommendations for antimicrobial use in febrile neutropenia in Japan: executive summary. Clin Infect Dis 39:S49–S52. https://doi.org/10.1086/383054
Yoshida M, Akiyama N, Fujita H, Miura K, Miyatake JI, Handa H, Kito K, Takahashi M, Shigeno K, Kanda Y, Hatsumi N, Ohtake S, Sakamaki H, Ohnishi K, Miyawaki S, Ohno R, Naoe T (2011) Analysis of bacteremia/fungemia and pneumonia accompanying acute myelogenous leukemia from 1987 to 2001 in the Japan Adult Leukemia Study Group. Int J Hematol 93:66–73. https://doi.org/10.1007/s12185-010-0746-y
Gafter-Gvili A, Fraser A, Paul M, Vidal L, Lawrie TA, van de Wetering MD, Kremer LCM, Leibovici L, Cochrane Gynaecological, Neuro-oncology and Orphan Cancer Group (2012) Antibiotic prophylaxis for bacterial infections in afebrile neutropenic patients following chemotherapy. Cochrane Database Syst Rev 1:CD004386. https://doi.org/10.1002/14651858.CD004386.pub3
Hamilton BK, Copelan EA (2012) Concise review: the role of hematopoietic stem cell transplantation in the treatment of acute myeloid leukemia. Stem Cells 30:1581–1586. https://doi.org/10.1002/stem.1140
Syrjälä H, Ohtonen P, Kinnunen U et al (2010) Blood stream infections during chemotherapy-induced neutropenia in adult patients with acute myeloid leukemia: treatment cycle matters. Eur J Clin Microbiol Infect Dis 29:1211–1218. https://doi.org/10.1007/s10096-010-0984-1
Becker K, Heilmann C, Peters G (2014) Coagulase-negative staphylococci. Clin Microbiol Rev 27:870–926. https://doi.org/10.1128/CMR.00109-13
Muto C, Pokrywka M, Shutt K et al (2005) A large outbreak of Clostridium difficile-associated disease with an unexpected proportion of deaths and colectomies at a teaching hospital following increased fluoroquinolone use. Infect Control Hosp Epidemiol 26:273–280
Garnica M, Nouér SA, Pellegrino FLPC et al (2013) Ciprofloxacin prophylaxis in high risk neutropenic patients: effects on outcomes, antimicrobial therapy and resistance. BMC Infect Dis 13:356. https://doi.org/10.1186/1471-2334-13-356
Ministry of Health, Labor and Welfare (2015) Japan Nosocomial Infections Surveillance [in Japanese]. https://janis.mhlw.go.jp/report/open_report/2016/3/1/ken_Open_Report_201600.pdf Accessed on 25 Dec 2017
Ministry of Health, Labor and Welfare (2005) Japan Nosocomial Infections Surveillance [in Japanese]. https://janis.mhlw.go.jp/report/season/nenpou/2005/ken_note.html Accessed on 25 Dec 2017
Acknowledgements
This work was supported in part by a grant from the Japan Adult Leukemia Study Group.
Author information
Authors and Affiliations
Consortia
Contributions
Hideaki Kato: research design and performance, data collection and interpretation, and manuscript writing. Hiroyuki Fujita: research organization and supervision. Nobu Akiyama: data analysis. Shun-ichi Kimura, Nobuhiro Hiramoto, Naoko Hosono, Tsutomu Takahashi, Kazuyuki Shigeno, Hitoshi Minamiguchi, Junichi Miyatake, Hiroshi Handa, Yoshinobu Kanda, Minoru Yoshida, Shuichi Miyawaki, Shigeki Ohtake, Tomoki Naoe, Hitoshi Kiyoi, Itaru Matsumura, and Japan Adult Leukemia Study Group: research performance. Yasushi Miyazaki: research supervision. For a complete list of the members of the JALSG, see the supplemental Appendix.
Corresponding author
Ethics declarations
Conflicts of interest
Hideaki Kato received grants from Japan Blood Products Organization and Shionogi. Hiroyuki Fujita received honoraria from Novartis, and payment for manuscripts from Chugai Pharmaceutical. Shun-ichi Kimura received honoraria from Pfizer, Astellas, Sumitomo Dainippon Pharma, and Nippon Kayaku. Tsutomu Takahashi received grants from Chugai Pharmaceutical, Kyowa Hakko Kirin, and Astellas, honoraria from Chugai Pharmaceutical, Kyowa Hakko Kirin, Taiho Pharmaceutical and Celgene Corporation. Junichi Miyatake received honoraria from Kyowa Hakko Kirin, Celgene Corporation, Sumitomo Dainippon Pharma, Takeda Pharmaceutical, and Chugai Pharmaceutical. Hiroshi Handa received consulting fee from Takeda Pharmaceutical and Celgene Corporation, grants and honoraria from Celgene Corporation, Takeda Pharmaceutical, Pfizer, Astellas, Sanofi, MSD, Shionogi, Daiichi Sankyo, Kyowa Hakko Kirin, Novartis, and Shire. Shunichi Miyawaki received a consulting fee from Astellas and expert testimony from Ohtsuka Pharmaceutical and Novartis. Hitoshi Kiyoi received consulting fees from Astellas and Daiichi Sankyo, grants from Chugai Pharmaceutical, Bristol-Myers Squibb, Kyowa Hakko Kirin, Zenyaku Kogyo, FUJIFILM Corporation, Nippon Boehringer Ingelheim, Astellas and Celgene Corporation, and honoraria from Bristol-Myers Squibb and Pfizer. Other authors: none to declare.
Electronic supplementary material
ESM 1
(DOCX 18 kb)
Rights and permissions
About this article
Cite this article
Kato, H., Fujita, H., Akiyama, N. et al. Infectious complications in adults undergoing intensive chemotherapy for acute myeloid leukemia in 2001–2005 using the Japan Adult Leukemia Study Group AML201 protocols. Support Care Cancer 26, 4187–4198 (2018). https://doi.org/10.1007/s00520-018-4292-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-018-4292-0