Abstract
In this paper, we present microfluidic chip platforms to enable electrophysiological measurements of nanometer-sized extracellular vesicles. The basis of the chip platform is the realization of a synthetic free-standing lipid bilayer spanned within a microfabricated aperture. To allow ion channel current measurements, the background current noise level should be reduced to a minimum. This can be realized by coating microfabricated apertures from silicon, silicon oxide, or silicon nitride with PTFE or Parylene. Three promising chip platform designs are presented. Electrophysiological measurements conducted with these microfluidic systems show gating events of membrane proteins fused into synthetic lipid bilayers.
Zusammenfassung
In diesem Beitrag präsentieren wir mikrofluidische Chip-Plattformen, um mit elektrophysiologischen Messungen nanometergroße extrazelluläre Vesikel zu messen. Die Grundlage der Chip-Plattform ist die Realisierung einer synthetischen freistehenden Lipid-Doppelschicht, die über eine mikrostrukturierte Öffnung gespannt ist. Um die Spannung im Ionenkanal zu messen, muss der Hintergrund-Geräuschpegel möglichst gering sein. Dies wird durch das Beschichten der mikrostrukturierten Öffnung aus Silikon, Silikon-Oxid oder Silikon-Nitrid mit PTFE oder Parylene erreicht. Drei erfolgversprechende Chip-Plattform-Entwürfe werden beschrieben. Elektrophysiologische Messungen, die mit diesen mikrofluidischen Systemen ausgeführt wurden, zeigen ein Kanalschaltverhalten, bei dem Membran-Proteine zu synthetischen Lipid-Doppelschichten verschmolzen werden.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Synthetic lipid bilayers are an attractive medium to investigate properties of biological cell membrane parts and its components. The membrane of biological cells plays a crucial role in various functions supporting the survival of the cell, a cell population, or even the whole organism [1]. To protect the cell from its environment and to ensure nutrient uptake, a complex system of regulated passive and active pore proteins are incorporated into the bilayer membrane. For example, the global struggle with antibiotic resistant bacteria is strongly related to biological membranes [2, 3]. Pore proteins and efflux-pumps located in the membrane of bacteria are responsible for the translocation of nutrients and blocking of harmful substances [4, 5]. The large variety of pore proteins, the regulation of the open and close stages of the pores, the heterogeneity of bacteria populations, and the short doubling times of bacteria make it very challenging to inhibit the growth of these fast adapting populations. Cell membranes also play an important role in cell-to-cell communication. Extracellular vesicles released from prokaryotic and eukaryotic cells are able to transport RNA, DNA, proteins and lipids to other cells [6, 7]. This information exchange can promote cancer progression (exchange of RNA) [6], or it can make bacteria resistant to specific drugs (exchange of pore proteins and efflux-pumps) [5].
Electrophysiological methods are a tool that can be applied to investigate the functionality of membrane components. Determination of charged particle transport through the membrane helps to further understand cell proliferation and disease development. Electrophysiological measurements recording the current flow of electrolyte or other charged particle translocation over the membrane gives insight about the opening and closing stages of pore proteins.
For mammalian cells (typically between 8 µm and 50 µm in diameter), ion channel current measurements can be conducted using a patch-clamp system [8, 9]. Here, a part of the membrane is patched with a pipette tip filled with an electrolyte solution and clamped with a set of electrodes to observe changes in the current. The diameter of the patched area is in the single micrometer range. By fixing the sample and utilizing micromanipulators, the patch can be realized.
Patch-clamping is quite challenging for much smaller biological samples such as bacteria, extracellular vesicles (EVs), or cell organelles. Other methods are required to investigate the electrophysical properties of these small particles.
One of the key challenges is to realize a controlled environment in which membrane proteins or patches of native bacterial lipids containing the proteins can be investigated. A solution that enables such investigations is by creating synthetic bilayer lipid membranes (BLM) and functionalize them by fusing biological particles (purified proteins, outer-membrane-vesicles (OMVs), or extracellular vesicles) into the BLM [10,11,12].
There are various microfabricated chip-based methods to realize synthetic lipid bilayer membranes. One option is to construct the bilayer lipids on a solid support [13, 14]. Also, tethered or cushioned BLMs were investigated [15, 16]. Such BLMs, placed on an indium tin oxide electrode covered by a polyaniline and poly acrylic acid film are very suitable to for atomic force microscopy investigations [17]. For electrophysiological membrane investigations, free-standing lipid bilayers are the most promising solution [18].
In this work, microfabricated chip platforms for the study of EVs and membrane pore proteins are discussed.
2 Aperture shape and material selection
The main advantage of microfluidic devices with free-standing BLMs is that the membrane is accessible from both sides. This is not the case for lipid bilayer systems that are on one side supported by a solid. Free-standing BLM platforms allow the use and integration of sensors on both sides of the membrane. They also allow measurements that require asymmetric buffer solutions (electrolyte or pH difference across the membrane). In addition, in this setup it is easy to replace or adjust the buffer solution at each side of the BLM.
The shape of the aperture and selected materials are important parameters for realizing a stable and thin membrane. The membrane should be free of lipid clogs and should not contain an excess of solvent. The presence of solvents such as n-decane and n-hexane in the hydrophobic region of the BLM does stabilize the membrane but unfortunately, also makes the membrane thick. In addition, solvents also negatively act on the functionality or even denature the pore proteins under investigation [19]. High-quality lipid bilayers spanned within an aperture should have a thickness of about twice the length of the selected phospholipid and should be free of excess solvent. For the commonly used synthetic pure lipid DPhPC (1,2-diphytanoyl-sn-glycerol-3-phospho-choline) the thickness of the bilayer at 20 °C is approximately 3.6 nm [20] and the permittivity of the hydrophobic chain approximately 2.2 [21]. Assuming that a lipid bilayer acts as a parallel plate capacitor, a rough estimation of the capacitance of a good quality 100 µm diameter DPhPC bilayer membrane amounts to 42 pF according to:
where A is the area of the membrane and d its thickness. A method to determine the quality or thickness is by applying a triangular voltage over the membrane and record the responding current trace.
The material specifications of the microfluidic system are crucial to construct stable BLMs. The system should be compatible with the aquatic based electrolyte and hydrophobic lipophilic nature of the lipid chains. The lipids should only form a (bi)layer over the aperture, and should not stick at the walls of the channels or reservoirs. Among other materials suitable for the fabrication of the aperture, PTFE is the most used material for constructing BLMs. PTFE is hydrophobic and after pre-treatment with n-hexadecane, also lipophilic [22].
There are different methods to construct free-standing BLMs in a micro-aperture. Painting [23], monolayer folding [10, 24], tip-dip [25], pseudo painting/air bubble bursting [26], and inkjet printing [27] are the most common methods. A graphical overview about the painting, monolayer folding, and tip-dip procedure is depicted in [28, 29]. For microfluidic devices with apertures surrounded by a channel structure, the painting and monolayer folding techniques are the most compatible. When there is more space available around the aperture, like in the 3d-printed reservoir system presented in [30], the pseudo painting/air bubble bursting method is highly suitable. With this method, stable BLMs can be realized within a few seconds, containing almost no protein hindering solvent (Fig. 1).
3 Microfluidic aperture system designs
In [31,32,33], the stability of free-standing BLMs painted in a microfabricated tapered shaped silicon-nitride aperture was investigated. Both sides of the silicon-nitride layer and supporting silicon were covered with silicon-dioxide. The apertures have a diameter of 20–30 µm and a thickness of 240 nm. Compared to straight etched apertures, the tapered shape (obtained by isotropic etching) has a smaller aspect ratio (diameter versus side wall length) and therefore, a smaller annulus disc and less stress around the lipid bilayer. It also requires less solvent in the hydrophobic part of the membrane because the annulus is less thick. This makes the tapered aperture better suited for pore protein investigations. With this system membranes were created with a lifetime of 45 h for plain BLMs, and a lifetime of 15 h for BLMs with fused gramicidin pore proteins.
Further iterations of the tapered aperture design were made to reduce the background current noise ([33]; Fig. 2a). This was achieved by adding an insulation layer on top of the silicon layer (not on top of the tapered silicon-nitride). A layer of PTFE, silicon-dioxide, and a combination of the two have been tested. The averaged peak-to-peak current noise was reduced from 5–10 pA for uncoated devices, to 3–5 pA and 1–2 pA for devices coated with PTFE or silicon-dioxide and PTFE, respectively. In order to show the performance of the system, the determination of small amplitude and fast gating events, the peak-to-peak current noise is given instead of the rms current noise. With this device single-channel current events (ions from the buffer solution migrate through the pore protein by applying a DV voltage over the BLM) of fast, less than 0.5 ms, gating alamethicin were successfully recorded (Fig. 2b).
a Schematic of a BLM platform with an integrated tapered aperture design. b Gating events of alamethicin. Applied potential: −100 mV. Sampling rate: 20 kHz. Low-pass filter: 5 kHz. (Adapted from Ref [33])
An attractive alternative for insulating PTFE coatings is parylene [30, 34, 35]. In Fig. 3a, a schematic of a microfabricated system including a 10 µm thick parylene film with 45 µm diameter apertures is depicted. This sheet was bonded between mechanically structured PMMA substrates [34]. The functionality of the microfluidic device was tested by recording the ion channel activity of OprM fused with a DPhPC bilayer (Fig. 3b).
a A schematic of a BLM platform with a parylene aperture fixed in a PMMA substrate. b Gating events of OprM. Applied potential: 120 mV. Sampling rate: 50 kHz. Low-pass filter: 10 kHz. (Adapted from Ref [34])
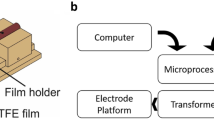
The advantage of parylene is that it is hydrophobic and lipophilic, and compared to PTFE does not require pre-treatment steps with solvents before constructing a free-standing membrane. Another advantage of parylene is that the deposition process yields a conformal coating of the microfabricated aperture. In Fig. 4a, a silicon-nitride aperture (thickness 500 nm) conformal coated with 10 µm parylene‑C is depicted [30, 36, 37]. These small robust apertures placed in a 3d-printed holder (Fig. 4b,c) with fluid reservoirs and Ag/AgCl electrode pairs are designed to be operated by an automated pipetting robot (9 mm pitch shown in Fig. 4b). Combining standardized high throughput screening technology with functionalized synthetic membrane systems will have practical advantages for membrane translocation investigations.
A BLM platform with a silicon nitride aperture, conformal coated with parylene. a Schematic of the aperture design. b Photo of the 3d-printed BLM platform. The insert shows a picture of the aperture chip (Chip dimension is 5 × 5 mm2). c A schematic of the BLM platform with a tilted aperture chip (yellow part). (Adapted from Ref [36])
To demonstrate the performance of a 90 µm diameter aperture coated with Parylene‑C, EVs overexpressing the membrane protein OmpF where introduced in one of the fluid reservoirs. In Fig. 5, the successful incorporation of EVs is shown by the occurring current events due to ion transport through open OmpF pores.
Gating events of OmpF incorporated into a BLM by fusion of extracellular vesicles released by E. coli. The vesicles where suspended in 1 M KCl buffer. Applied potential: 150 mV. Sampling rate: 10 kHz. Low-pass filter: 1 kHz. The current events (insert) represent ion transport through opened OmpF pores. (Adapted from Ref [37])
4 Conclusions
In conclusion, there are various methods to realize functionalized free-standing synthetic lipid bilayer systems. Microfabricated aperture designs are because of their geometry accuracy, aspect ratio, easy accessibility from both membrane sides, and fabrication reproducibility very suitable for membrane translocation investigations. The material and shape of the aperture play an important role in realizing stable and thin BLMs. Microfluidic based aperture systems show a promising basis to be further developed towards high-throughput automated membrane translocation platforms for extracellular vesicles investigations.
References
Brito C, Cabanes D, Sarmento Mesquita F, Sousa S (2019) Mechanisms protecting host cells against bacterial pore-forming toxins. Cell Mol Life Sci 76:1319–1339. https://doi.org/10.1007/s00018-018-2992-8
Delcour AH (2009) Outer membrane permeability and antibiotic resistance. Biochim Biophys Acta 1794:808–816. https://doi.org/10.1016/j.bbapap.2008.11.005
Hiller CX, Hübner U, Fajnorova S, Schwartz T, Drewes JE (2019) Antibiotic microbial resistance (AMR) removal efficiencies by conventional and advanced wastewater treatment processes: A review. Sci Total Environ 685:596–608. https://doi.org/10.1016/j.scitotenv.2019.05.315
Choi U, Lee CR (2019) Distinct roles of outer membrane porins in antibiotic resistance and membrane integrity in Escherichia coli. Front Microbiol 10:1–9. https://doi.org/10.3389/fmicb.2019.00953
Sun J, Deng Z, Yan A (2014) Bacterial multidrug efflux pumps: Mechanisms, physiology and pharmacological exploitations. Biochem Biophys Res Commun 453:254–267. https://doi.org/10.1016/j.bbrc.2014.05.090
O’Brien K, Breyne K, Ughetto S, Laurent LC, Breakefield XO (2020) RNA delivery by extracellular vesicles in mammalian cells and its applications. Nat Rev Mol Cell Biol 21:585–606. https://doi.org/10.1038/s41580-020-0251-y
Tkach M, Théry C (2016) Communication by extracellular vesicles: Where we are and where we need to go. Cell 164:1226–1232. https://doi.org/10.1016/j.cell.2016.01.043
Neher E, Sakmann B (1976) Single-channel currents recorded from membrane of denervated frog muscle fibres. Nature 260:799–802. https://doi.org/10.1038/260799a0
Sakmann B, Neher E (1984) Patch clamp techniques for studying ionic channels in excitable membranes. Annu Rev Physiol 46:455–472. https://doi.org/10.1146/annurev.ph.46.030184.002323
Montal M, Mueller P (1972) Formation of bimolecular membranes from lipid monolayers and a study of their electrical properties. Proc Natl Acad Sci USA 69:3561–3566. https://doi.org/10.1073/pnas.69.12.3561
Wang J, Terrasse R, Bafna JA, Benier L, Winterhalter M (2020) Electrophysiological characterization of transport across outer-membrane channels from gram-negative bacteria in presence of lipopolysaccharides. Angew Chem Int Ed 59:8517–8521. https://doi.org/10.1002/anie.201913618
Ahmed T, Bafna JA, Hemmler R, Gall K, Wagner R, Winterhalter M, Vellekoop MJ, van den Driesche S (2022) Silicon nitride-based micro-apertures coated with parylene for the investigation of pore proteins fused in free-standing lipid bilayers. Membranes (Basel). https://doi.org/10.3390/membranes12030309
Ferhan AR, Yoon BK, Park S, Sut TN, Chin H, Park JH, Jackman JA, Cho N‑J (2019) Solvent-assisted preparation of supported lipid bilayers. Nat Protoc 14:2091–2118. https://doi.org/10.1038/s41596-019-0174-2
van Weerd J, Karperien M, Jonkheijm P (2015) Supported lipid Bilayers for the generation of dynamic cell-material interfaces. Adv Healthcare Mater 4:2743–2779. https://doi.org/10.1002/adhm.201500398
Arslan Yildiz A, Yildiz UH, Liedberg B, Sinner EK (2013) Biomimetic membrane platform: Fabrication, characterization and applications. Colloids Surf B Biointerfaces 103:510–516. https://doi.org/10.1016/j.colsurfb.2012.10.066
Sato J, Niiyama Y, Misawa N, Tero R (2019) Preparation of tethered-type supported lipid bilayer using water-soluble silane coupling agent. Jpn J Appl Phys 58:SIID5. https://doi.org/10.7567/1347-4065/ab1641
Ge C, Orosz KS, Armstrong NR, Saavedra SS (2011) Poly(aniline) nanowires in sol-gel coated ITO: A pH-responsive substrate for planar supported lipid bilayers. ACS Appl Mater Interfaces 3:2677–2685. https://doi.org/10.1021/am2004637
Ren X, Liu K, Zhang Q, Noh H, Kumbur EC, Yuan WW, Zhou JG, Chong PLG (2014) Design, fabrication, and characterization of archaeal tetraether free-standing planar membranes in a PDMS-and PCB-based fluidic platform. ACS Appl Mater Interfaces 6:12618–12628. https://doi.org/10.1021/am502613x
Benz R, Fröhlich O, Läuger P, Montal M (1975) Electrical capacity of black lipid films and of lipid bilayers made from monolayers. Biochim Biophys Acta 394:323–334. https://doi.org/10.1016/0005-2736(75)90287-4
Kučerka N, Nieh MP, Katsaras J (2011) Fluid phase lipid areas and bilayer thicknesses of commonly used phosphatidylcholines as a function of temperature. Biochim Biophys Acta 1808:2761–2771. https://doi.org/10.1016/j.bbamem.2011.07.022
Simeonova M, Gimsa J (2006) The influence of the molecular structure of lipid membranes on the electric field distribution and energy absorption. Bioelectromagnetics 27:652–666. https://doi.org/10.1002/bem.20259
Takei T, Yaguchi T, Fujii T, Nomoto T, Toyota T, Fujinami M (2015) Measurement of membrane tension of free standing lipid bilayers via laser-induced surface deformation spectroscopy. Soft Matter 11:8641–8647. https://doi.org/10.1039/C5SM01264C
Tien HT, Wescott WC, Rudin DO, Mueller P (1962) Reconstitution of cell membrane structure in vitro and its transformation into an excitable system. Nature 194:979–980
White SH, Petersen DC, Simon S, Yafuso M (1976) Formation of planar bilayer membranes from lipid monolayers. A critique. Biophys J 16:481–489. https://doi.org/10.1016/S0006-3495(76)85703-7
Matsuno Y, Osono C, Hirano A, Sugawara M (2004) Single-channel recordings of gramicidin at agarose-supported bilayer lipid membranes formed by the tip-dip and painting methods. Anal Sci 20:1217–1221. https://doi.org/10.2116/analsci.20.1217
Braun CJ, Baer T, Moroni A, Thiel G (2014) Pseudo painting/air bubble technique for planar lipid bilayers. J Neurosci Methods 233:13–17. https://doi.org/10.1016/j.jneumeth.2014.05.031
Yamada M, Imaishi H, Morigaki K (2013) Microarrays of phospholipid bilayers generated by inkjet printing. Langmuir 29:6404–6408. https://doi.org/10.1021/la400570h
Hirano-Iwata A, Niwano M, Sugawara M (2008) The design of molecular sensing interfaces with lipid-bilayer assemblies. Trends Analyt Chem 27:512–520. https://doi.org/10.1016/j.trac.2008.04.006
Bartsch P, Walter C, Selenschik P, Honigmann A, Wagner R (2012) Horizontal bilayer for electrical and optical recordings. materials 5:2705–2730. https://doi.org/10.3390/ma5122705
Ahmed T, van den Driesche S, Bafna JA, Oellers M, Hemmler R, Gall K, Wagner R, Winterhalter M, Vellekoop MJ (2020) Rapid lipid bilayer membrane formation on parylene coated apertures to perform ion channel analyses. Biomed Microdevices 22:32. https://doi.org/10.1007/s10544-020-0473-y
Tadaki D, Yamaura D, Araki S, Yoshida M, Arata K, Ohori T, Ishibashi KI, Kato M, Ma T, Miyata R, Tozawa Y, Yamamoto H, Niwano M, Hirano-Iwata A (2017) Mechanically stable solvent-free lipid bilayers in nano- and micro-tapered apertures for reconstitution of cell-free synthesized hERG channels. Sci Rep 7:17736. https://doi.org/10.1038/s41598-017-17905-x
Hirano-Iwata A, Aoto K, Oshima A, Taira T, Yamaguchi R, Kimura Y, Niwano M (2010) Free-standing lipid bilayers in silicon chips-membrane stabilization based on microfabricated apertures with a nanometer-scale smoothness. Langmuir 26:1949–1952. https://doi.org/10.1021/la902522j
Oshima A, Hirano-Iwata A, Nasu T, Kimura Y, Niwano M (2012) Mechanically stable lipid bilayers in teflon-coated silicon chips for single-channel recordings. Micro Nanosyst 4:2–7. https://doi.org/10.2174/1876402911204010002
Wang W, Monlezun L, Picard M, Benas P, Franais O, Broutin I, le Pioufle B (2012) Activity monitoring of functional OprM using a biomimetic microfluidic device. Analyst 137:847–852. https://doi.org/10.1039/c2an16007b
Suzuki H, le Pioufle B, Takeuhci S (2009) Ninety-six-well planar lipid bilayer chip for ion channel recording Fabricated by hybrid stereolithography. Biomed Microdevices 11:17–22. https://doi.org/10.1007/s10544-008-9205-4
Ahmed T, Bafna JA, Hemmler R, Gall K, Wagner R, Winterhalter M, Vellekoop MJ, van den Driesche S (2022) Silicon nitride-based micro-apertures coated with parylene for the investigation of pore proteins fused in free-standing lipid bilayers. Membranes (Basel) 12:309. https://doi.org/10.3390/membranes12030309
Ahmed T, van den Driesche S, Arun Bafna J, Oellers M, Hemmler R, Gall K, Wagner R, Winterhalter M, Vellekoop MJ (2019) Parylene‑C coated micro-apertures with painted synthetic lipid bilayer membranes for the investigation of outer-membrane-vesicle fusion. In: 2019 IEEE SENSORS, IEEE, pp 1–4 https://doi.org/10.1109/SENSORS43011.2019.8956698
Acknowledgements
We thank Mathias Winterhalter and Richard Wagner (Jacobs University, Bremen, Germany), Karsten Gall and Roland Hemmler (Ionovation GmbH, Bissendorf, Germany) and Tanzir Ahmed (IMSAS, University of Bremen) for their cooperation in lipid bilayer research.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
van den Driesche, S., Vellekoop, M.J. Chip platforms with synthetic lipid bilayers for electrophysiological analyses of pore proteins and extracellular vesicles. Elektrotech. Inftech. 139, 471–476 (2022). https://doi.org/10.1007/s00502-022-01045-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00502-022-01045-w