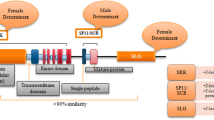
Abstract
Key message
S29 haplotype does not require the MLPK function for self-incompatibility in Brassica rapa.
Abstract
Self-incompatibility (SI) in Brassicaceae is regulated by the self-recognition mechanism, which is based on the S-haplotype-specific direct interaction of the pollen-derived ligand, SP11/SCR, and the stigma-side receptor, SRK. M locus protein kinase (MLPK) is known to be one of the positive effectors of the SI response. MLPK directly interacts with SRK, and is phosphorylated by SRK in Brassica rapa. In Brassicaceae, MLPK was demonstrated to be essential for SI in B. rapa and Brassica napus, whereas it is not essential for SI in Arabidopsis thaliana (with introduced SRK and SP11/SCR from related SI species). Little is known about what determines the need for MLPK in SI of Brassicaceae. In this study, we investigated the relationship between S-haplotype diversity and MLPK function by analyzing the SI phenotypes of different S haplotypes in a mlpk/mlpk mutant background. The results have clarified that in B. rapa, all the S haplotypes except the S29 we tested need the MLPK function, but the S29 haplotype does not require MLPK for the SI. Comparative analysis of MLPK-dependent and MLPK-independent S haplotype might provide new insight into the evolution of S-haplotype diversity and the molecular mechanism of SI in Brassicaceae.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Self-incompatibility (SI) is controlled by a single locus, called the S locus, with highly polymorphic multiple alleles (Bateman 1955). In Brassicaceae, the S-locus region contains two genes, stigma and pollen recognition determinant genes, S receptor kinase (SRK) and S-locus protein 11 (SP11, also called SCR), respectively (Stein et al. 1991; Takasaki et al. 2000; Suzuki et al. 1999; Schopfer et al. 1999; Takayama et al. 2000; reviewed in Watanabe et al. 2012; Fujii and Takayama 2018; Abhinandan et al. 2021). S-haplotype-specific direct interaction of SRK and SP11 causes phosphorylation of SRK and induces self-pollen rejection (Takayama et al. 2001; Shimosato et al. 2007; Murase et al. 2020). Because the SRK and SP11 genes are inherited as a single segregational unit, S alleles are termed S haplotypes (Nasrallah and Nasrallah 1993). S-haplotype diversity is determined by sequence polymorphism of SRK and SP11 genes in the S locus and over 100 have been identified (S1, S2, S3, …, Sn) in the genus Brassica (Nou et al. 1993; Sakamoto and Nishio 2001; Watanabe et al. 2000; Ockendon 1982; Charlesworth et al. 2003; Paetsch et al. 2006), and over 30 in Raphanus (Kim and Kim 2019; Fukushima et al. 2021). In Brassica, S haplotypes are classified into two classes (class-I and -II) based on the sequence similarity of the extracellular domain encoded by SRK (and its homologous gene termed SLG). The pollen-side SI phenotype is almost co-dominant between class-I S haplotypes, and the stigma-side is also co-dominant with a few exceptions (Hatakeyama et al. 1998b). On the other hand, class-II S haplotypes are generally recessive to class-I S haplotypes in pollen, but the two classes are co-dominant in the stigma (Hatakeyama et al. 1998b). Diploid plants carrying two co-dominant S haplotypes exhibit SI specificity of both S haplotypes encoded by the parental genome. The regulation of SP11 gene expression controls this dominance relationship on the pollen side through epigenetic mechanisms, including small RNA-mediated DNA methylation (Shiba et al. 2002; Kakizaki et al. 2003; Tarutani et al. 2010; Yasuda et al. 2016).
Studies of the molecular mechanism of SI in Brassicaceae have been conducted mainly in Brassica species (Brassica rapa, Brassica oleracea, Brassica napus). However, in Arabidopsis thaliana, a naturally self-compatible species, it has been reported that SI can be imparted by introducing SRK and SP11 genes of closely related SI species (Arabidopsis lyrata, Arabidopsis halleri) (Nasrallah et al. 2002; Zhang et al. 2019; Fujii et al. 2020). In addition, it has been reported that a change from self-compatibility (SC) to SI occurs by restoring the inversion of the SP11 gene in A. thaliana (Tsuchimatsu et al. 2010). This suggests that the components required for the SI reaction are common to Brassica and Arabidopsis species.
In the Brassica SI system, downstream signaling pathways and the target of SRK leading to self-pollen rejection are becoming better understood. The M-locus protein kinase (MLPK), which was isolated by positional cloning of the M locus of the self-compatible B. rapa variety ‘yellow sarson’, is an essential positive regulator of the SI response (Murase et al. 2004). MLPK belongs to the receptor-like cytoplasmic kinase family. Biochemical analysis revealed that MLPK is a membrane-bound kinase present in the cell membrane fraction of the stigma, the N-terminal myristoylation motif is involved in membrane localization (Murase et al. 2004), and MLPK directly interacts with and is phosphorylated by SRK (Kakita et al. 2007). Because MLPK-deficient plants exhibit a completely SC phenotype in B. rapa and B. napus, MLPK is considered to be an essential protein that positively regulates the self-incompatibility signaling system in Brassica (Murase et al. 2004; Chen et al. 2019). On the other hand, it has been reported that mutation of APK1b, the gene in A. thaliana that shares the highest similarity to MLPK, does not affect the SI response of self-incompatible SRK/SP11 transgenic A. thaliana (Kakita et al. 2007; Kitashiba et al. 2011), and APK1b has been shown to be involved in light-induced stomatal opening in A. thaliana (Elhaddad et al. 2014). According to the analysis by Azibi et al. (2020), there are 3 copies of MLPK in the genus Brassica, which have arisen by whole-genome triplication (WGT), and in the phylogenetic analysis, the gene closest to APK1b in B. rapa is one of the paralogues of MLPK, not MLPK itself. Thus, the recruitment of MLPK in SI signaling in Brassica may be the result of neo-functionalization of the duplicated genes, which occurred after the WGT in the origin of Brassica species (Azibi et al. 2020).
In this study, we investigated the genetic association of MLPK with the SI signaling of different S haplotypes in B. rapa. We found that the S29 haplotype of B. rapa does not require MLPK functionality in SI, unlike other S haplotypes.
Materials and methods
Plant materials and test pollination
Plant materials used in this study are listed in Supplementary Table S1. As the mlpk/mlpk mutant donor line, we used S8/S8, mlpk/mlpk (hereafter referred to as S8S8, mm) from Murase et al. (2004). The 11 S homozygous lines of B. rapa used in the present experiment were selected from those established by Nou et al. (1993) containing two different populations, from Oguni in Japan and from Balcesme in Turkey. In addition to these 11 lines, one S homozygous line (S60), which was derived from a Japanese commercial hybrid variety (cv. Osome, Takii & Co., Ltd, Takasaki et al 1999), was also used in this study. All S homozygous lines have been confirmed to exhibit the self-incompatible phenotype (Nou et al. 1993; Takasaki et al 1999). Pollination phenotype was determined by test cross as described in Takada et al. (2017). Pollinated stigmas were stained with aniline blue and observed by UV fluorescence microscopy (Zeiss Axio Imager A2, Kho and Bear 1968). The degree of compatibility (compatibility score; CS) in each test pollination was scored on a five-point scale based on pollen tube penetration as follows: (CS = 5) penetration of more than 10 pollen tubes into the style; (CS = 4) penetration of 1–10 pollen tubes into the style; (CS = 3) penetration of pollen tubes into papilla cells but not into the style; (CS = 2) germination of pollen but no pollen-tube penetration into papilla cells; (CS = 1) no germination of pollen. Average scores less than 3 were defined as incompatible, and scores 3 and above as compatible (Takada et al. 2017; 2021). Stigmas from at least three flowers for each cross combination were tested and this was replicated on at least five different dates.
Determination of the S haplotype and MLPK genotype
The S haplotype of each plant was determined by polymerase chain reaction (PCR). Total DNA was extracted from young leaf tissue of B. rapa using DNeasy plant mini kit (Qiagen). PCR was performed using ExTaq DNA polymerase (Takara Bio). To determine the S haplotype of each plant, class I/class II-specific PCR primers for amplification of SLG were used, as described in Nishio et al. (1996). The each SP11 genes were amplified by using S-haplotype-specific SP11 primer sets (Supplementary Table S2). For genotyping of MLPK, the functional MLPK allele was specifically amplified using the PCR primers wtMLPK-F and wtmMLPK-R, and the mutated mlpk allele was specifically amplified using the mMLPK-F and wtmMLPK-R primers (Takada et al. 2013). The PCR product was subjected to electrophoresis on a 1% agarose gel. For isolation of MLPK genomic sequences from the S29 haplotype, PCR amplification using a primer pair MLPKF and MLPKR was performed, and amplified fragments were cloned into pTAC2 vector (Biodynamics Laboratory Inc.). The nucleotide sequence was determined with a 3500 Genetic Analyzer using Big Dye Terminator version 3.1 Cycle Sequencing Kit (Applied Biosystems).
Sequence and multiple alignment analysis
Accession numbers of SRK sequences used in amino acid alignment analysis are listed in Supplementary Table S3. GENETYX version 13 software package (GENETYX Corp.) was used for the sequence comparison and alignment.
Results and discussion
The breakdown of SI with S8 haplotype in mm mutant background has been reported previously (Murase et al. 2004; Fukai et al. 2001). We tested the SC phenotype of S8S8, mm (S8/S8, mlpk/mlpk) homozygous plants, established by Murase et al. (2004). Self-pollination of 11 S8S8, mm homozygous plants showed SC phenotype, and the test cross between the stigma of 34 S8S8, mm homozygous plants and the pollen from S8S8, MM tester line resulted in compatible pollination (Table 1, Fig. 1A). To analyze details of the genetic relationship between S haplotype diversity and MLPK function in the SI of Brassica, we established mm mutant lines possessing the different S-haplotype backgrounds by crossing S8S8, mm homozygous SC plants with 7 class-I and 4 class-II S haplotypes (Table 1, 2, Supplementary Table S1). Each S haplotype and MM homozygous plant was crossed with S8S8, mm plants. The mm plants in each S haplotype were selected from the F2 segregating population. The obtained mm homozygous plants were test crossed with the pollen from S homozygous tester lines (Supplementary Table S1). The S12 and S24 haplotypes have the same SI recognition identity but originated from different locations (S12 from Japan, S24 from Turkey, Nou et al. 1993; Matsushita et al. 1996; Takada et al. 2017). In the class-I S haplotypes (S12, S24, S21, S25, S27, S35, S37, and S45) examined in this study, all individuals with the mm homozygous background showed compatibility phenotype on the stigma side, indicating that MLPK is essential for SI in these class-I haplotypes (Table 1, Fig. 1B-E). We observed fully germinated and penetrated pollen tubes in each compatible cross (Fig. 1B-E).
Representative photographs of test crosses. Photographs were obtained by UV fluorescence microscopy (a-l). a Cross pollination of S8S8, mm stigma with S8S8 tester pollen (♀S8S8, mm × ♂S8S8). b ♀S12S12, mm × ♂S12S12, c ♀S21S21, mm × ♂S8S21, d ♀S25S25, mm × ♂S25S25, e ♀S27S27, mm × ♂S27S27, f ♀S40S40, mm × ♂S40S40, g ♀S60S60, mm × ♂S60S60, h ♀S29S29, MM × ♂S29S29, i) ♀S29S29, mm × ♂S29S29, j ♀S29S8, mm × ♂S8S8, k ♀S29S8, mm × ♂S29S29, (l) S29S8, mm self-pollination. MLPK genotype of pollen donors was MM in cross-pollinations and mm in self-pollinations. Scale bars show 100 μm
Among 4 class-II S haplotypes (S29, S40, S44, and S60) reported in Kakizaki et al. (2003), S40, S44, and S60 haplotypes with mm exhibited SC phenotype (compatibility with the pollen from plants possessing the same S haplotype) as in the class-I S-haplotypes (Table 2, Fig. 1F, G), indicating that the function of MLPK is required for SI recognition and reaction in these 3 class-II S haplotypes (S40, S44, and S60). However, the S29 haplotype with mm unexpectedly exhibited the SI reaction, which could not be explained by the current theory of Brassica self-incompatibility (Table 2, Fig. 1H, I). The stigma of 8 mlpk mutant plants with the S29 allele (S29S29, mm) showed SI phenotype and incompatibility with the pollen from S29S29 tester plants (Table 2, Fig. 1I). We could not distinguish the SI phenotype of S29S29, mm plants and the SI of wild type S29S29, MM plant. This result is the first detection of an MLPK-independent SI phenotype in the genus Brassica.
To further improve the experiments in Table 1 with limited plant number, and to test the MLPK function in S-heterozygous plants, we selected mm mutant plants from lines heterozygous for S8 haplotype and 7 other S haplotypes (S24, S25 S45, S29, S40, S44 and S60) and checked the pollination phenotype (Table 3). The stigma-side dominant relationship of all S-haplotype combinations used in this study have been reported as co-dominant, except S8S60 heterozygous plants (Hatakeyama et al. 1998b). The stigma-side co-dominant relationship of S8S60 heterozygous plants was determined in this study (data not shown). All the combinations except S8S29 exhibited compatible phenotype with the pollen from its own S haplotype (Table 3). When we checked the pollination phenotype of 22 S8S29 heterozygous plants (S8S29, mm), all 22 plants showed compatibility with the pollen from S8S8 plants and incompatibility with the pollen from S29S29 plants (Table 3, Fig. 1J-L). It is interesting that the SI signal in SRK29 appears to be retained although the SRK8-mediated signal transduction appears to have been lost by mlpk mutation in the S8S29, mm plants.
Moreover, the complete genetic linkage between the S locus with S29 haplotype and the MLPK-independent SI phenotype suggests that the S29 haplotype does not require MLPK for the SP11/SRK-based SI signal transduction, rather than the existence of other genetic factor(s), which can complement the mlpk mutation, in the vicinity of S locus of the S29 haplotype (Table 2, 3). One of the three duplicated MLPK-like genes, A07p21240.2.BraZ1(B. rapa Z1 ver. 2, Istace et al. 2021), is located on chromosome A07 where the S-locus resides, but the two loci are far apart (Azibi et al 2020). Therefore, it is unlikely that the A07p21240.2.BraZ1 gene on chromosome A07 of the S29 haplotype has overlapping functions with MLPK. It is commonly inferred that the MLPK dependence of the SI mechanism is associated with direct or indirect binding of MLPK to the SRK or SP11-SRK complex, phosphorylation of MLPK by SRK, or both. Here, we isolated and sequenced the MLPK gene from the S29 haplotypes, and we could not find any difference at the nucleotide level between S29 and S8 haplotypes (data not shown, the MLPK sequence in the S8 haplotype has been reported in Murase et al. 2004). To clarify the specific amino acid sites of SRK29, especially in the intracellular region (considered to bind with MLPK), we compared amino acids sequences of SRK from class-II S haplotypes (Fig. 2, Supplementary Fig. S1). The genetic diversity of SRK gene in class-II S haplotypes is reported to be lower than class-I S haplotypes (Hatakeyama et al 1998a). The result showed that only five amino acids are specific to SRK29, compared with three other SRKs (SRK40, SRK44, and SRK60, Fig. 2, Supplementary Fig. S1). Further biochemical and genetic experiments are needed, but these five amino acids may determine MLPK dependence in B. rapa.
In this study, we revealed that all S-haplotypes except the S29 haplotype have a MLPK-dependent SI system. Furthermore, we also showed that the S29 haplotype does not require MLPK for the SI system. Because there have been reports that MLPK is a co-factor for SRK kinase function, it is likely that SRK alone transduces the phosphorylation signal to the downstream target of SI response in the S29 haplotype (Murase et al. 2004; Kakita et al. 2007; Chen et al. 2019). The S29 haplotype of B. rapa has been characterized as the most recessive in the pollen-side dominance relationship (Hatakeyama et al. 1998b; Kakizaki et al. 2003). It is interesting to consider whether there is any relationship between the dominance relationship and the discovery of the MLPK-independent SI system in B. rapa. Two hypotheses can be proposed: one is that the S29 haplotype is the ancestral S haplotype in Brassica, which does not need MLPK, as in Arabidopsis, and the other is that the S29 haplotype has lost its MLPK dependence after it had previously been acquired. In either case, this study reveals that the SI signaling pathway does not require MLPK in the S29 haplotype of B. rapa, raising the further question of why many S haplotypes in B. rapa need MLPK. After the Arabidopsis-Brassica diversification and the significant reduction in the number of ancient S haplotypes, the S haplotype of Brassica is thought to have rapidly increased in diversity in a very limited time (Kusaba et al. 2001; Edh et al. 2009). It is interesting to consider that the acquisition of MLPK, as the intracellular co-receptor with SRK in the SI signaling pathway, contributed to this rapid spread of the S haplotype.
Author contribution statement
MO, YT and MW designed the experiments. MO, YT, YS and TO performed the experimental work and collected data. YT, KM, ST, GS and MW wrote the manuscript. All authors read and approved the manuscript.
References
Abhinandan K, Sankaranarayanan S, Macgregor S, Goring DR, Samuel MA (2021) Cell-cell signaling during the Brassicaceae self-incompatibility response. Trends Plant Sci 27:472–487. https://doi.org/10.1016/j.tplants.2021.10.011
Azibi T, Hadj-arab H, Lodé M et al (2020) Impact of whole genome triplication on the evolutionary history and the functional dynamics of regulatory genes involved in Brassica self-incompatibility signaling pathway. Plant Reprod 33:43–58. https://doi.org/10.1007/s00697-020-00385-x
Bateman AJ (1955) Self-incompatibility systems in angiosperms. III Cruciferae Heredity 9:53–68. https://doi.org/10.1038/hdy.1955.2
Charlesworth D, Bartolomé C, Schierup MH, Mable BK (2003) Haplotype structure of the stigmatic self-incompatibility gene in natural populations of Arabidopsis lyrata. Mol Biol Evo 20:1741–1753. https://doi.org/10.1093/molbev/msg170
Chen F, Yang Y, Li B et al (2019) Functional analysis of M-locus protein kinase revealed a novel regulatory mechanism of self-incompatibility in Brassica napus L. Int J Mol Sci 20:3303. https://doi.org/10.3390/ijms20133303
Edh K, Widen B (2009) The evolution and diversification of S-locus haplotypes in Brassicaceae family. Genetics 181:977–984. https://doi.org/10.1534/genetics.108.090837
Elhaddad NS, Hunt L, Sloan J, Gray JE (2014) Light-induced stomatal opening is affected by the guard cell protein kinase APK1b. PLoS ONE. https://doi.org/10.1371/journal.pone.0097161
Fujii S, Takayama S (2018) Multilayered dominance hierarchy in plant self-incompatibility. Plant Reprod 31:15–19. https://doi.org/10.1007/s00497-017-0319-9
Fujii S, Shimosato-Asano H, Kakita M et al (2020) Parallel evolution of dominant pistil-side self-incompatibility suppressors in Arabidopsis. Nat Commun 11:1404. https://doi.org/10.1038/s41467-020-15212-0
Fukai E, Nishio T, Nasrallah ME (2001) Molecular genetic analysis of the candidate gene for MOD, a locus required for self-incompatibility in Brassica rapa. Mol Genet Genomics 265:519–525. https://doi.org/10.1007/s004380000440
Fukushima K, Kanomata T, Kon A et al (2021) Spatiogenetic characterization of S receptor kinase (SRK) alleles in naturalized populations of Raphanus sativus L. var. raphanistroides on Yakushima island. Genes Genet Syst 96:1–11. https://doi.org/10.1266/ggs.20-0006
Hatakeyama K, Takasaki T, Watanabe M, Hinata K (1998a) Molecular characterization of S locus genes, SLG and SRK, in a pollen-recessive self-incompatibility haplotype of Brassica rapa L. Genetics 149:1587–1597. https://doi.org/10.1093/genetics/149.3.1587
Hatakeyama K, Watanabe M, Takasaki T, Ojima K, Hinata K (1998b) Dominance relationships between S-alleles in self-incompatible Brassica campestris L. Heredity 80:241–247. https://doi.org/10.1046/j.1365-2540.1998.00295.x
Istace B, Belser C, Falentin C et al (2021) Sequencing and chromosome-scale assembly of plant genomes, Brassica rapa as a use case. Biology 10:732. https://doi.org/10.3390/biology10080732
Kakita M, Murase K, Iwano M, Matsumoto T, Watanabe M, Shiba H, Isogai A, Takayama S (2007) Two distinct forms of M-locus protein kinase localize to the plasma membrane and interact directly with S-locus receptor kinase to transduce self-incompatibility signaling in Brassica rapa. Plant Cell 19:3961–3973. https://doi.org/10.1105/tpc.106.049999
Kakizaki T, Takada Y, Ito A, Suzuki G, Shiba H, Takayama S, Isogai A, Watanabe M (2003) Linear dominance relationship among four class-II S haplotype in pollen is determined by the expression of SP11 in Brassica self-incompatibility. Plant Cell Physiol 44:70–75. https://doi.org/10.1093/pcp/pcg009
Kho YO, Bear J (1968) Observing pollen tubes by means of fluorescence. Euphytica 17:298–302. https://doi.org/10.1007/BF00021224
Kim DS, Kim S (2019) Development of a new S locus haplotyping system based on three tightly linked genes in the S locus controlling self-incompatibility in radish (Raphanus sativus L.). Sci Hortic 243:70–77. https://doi.org/10.1016/j.scienta.2018.08.017
Kitashiba H, Liu P, Nishio T, Nasrallah JB, Nasrallah ME (2011) Functional test of Brassica self-incompatibility modifiers in Arabidopsis thaliana. Proc Natl Acad Sci USA 108:18173–18178. https://doi.org/10.1073/pnas.1115283108
Kusaba M, Dwyer K, Hendershot J, Vrebalov J, Nasrallah JB, Nasrallah ME (2001) Self-incompatibility in the genus Arabidopsis: Characterization of the S locus in the outcrossing A. lyrata and its autogamous relative A. thaliana. Plant Cell 13:627–643. https://doi.org/10.2307/3871411
Matsushita M, Watanabe M, Yamakawa S, Takayama S, Isogai A, Hinata K (1996) The SLGs corresponding to the same S24-haplotype are perfectly conserved in three different self-incompatible Brassica campestris L. Genes Genet Syst 71:255–258
Murase K, Shiba H, Iwano Che FS, Watanabe M, Isogai A, Takayama S (2004) A membrane-anchored protein kinase involved in Brassica self-incompatibility signalling. Science 303:1516–1519. https://doi.org/10.1126/science.1093586
Murase K, Moriwaki Y, Mori T et al (2020) Mechanism of self/nonself-discrimination in Brassica self-incompatibility. Nat Commun 11:4916. https://doi.org/10.1038/s41467-020-18698-w
Nasrallah JB, Nasrallah ME (1993) Pollen-stigma signaling in the sporophytic self-incompatibility response. Plant Cell 5:1325–1335. https://doi.org/10.2307/3869785
Nasrallah ME, Liu P, Nasrallah JB (2002) Generation of self-incompatible Arabidopsis thaliana by transfer of two S locus genes from A. lyrata. Science 297:247–249. https://doi.org/10.1126/science.1072205
Nishio T, Kusaba M, Watanabe M, Hinata K (1996) Registration of S alleles in Brassica campestris L by the restriction fragment size of SLGs. Theor Appl Genet 92:388–394. https://doi.org/10.1007/BF00223684
Nou IS, Watanabe M, Isogai A, Hinata K (1993) Comparison of S-alleles and S-glycoproteins between two wild populations of Brassica campestris in Turkey and Japan. Sex Plant Reprod 6:79–86. https://doi.org/10.1007/BF00227652
Ockendon DJ (1982) An S-allele survey of cabbage (Brassica oleracea var. capitata). Euphytica 31:325–331. https://doi.org/10.1007/BF00021647
Paetsch M, Mayland-Quellhorst S, Neuffer B (2006) Evolution of the self-incompatibility system in the Brassicaceae: identification of S-locus receptor kinase (SRK) in self-incompatible Capsella grandiflora. Heredity 97:283–290. https://doi.org/10.1038/sj.hdy.6800854
Sakamoto K, Nishio T (2001) Distribution of S haplotypes in commercial cultivars of Brassica rapa. Plant Breeding 120:155–161. https://doi.org/10.1046/j.1439-0523.2001.00575.x
Schopfer CR, Nasrallah ME, Nasrallah JB (1999) The male determinant of self-incompatibility in Brassica. Science 286:1697–1700. https://doi.org/10.1126/science.286.5445.1697
Shiba H, Iwano M, Entani T et al (2002) The dominance of alleles controlling self-incompatibility in Brassica pollen is regulated at the RNA level. Plant Cell 14:491–504. https://doi.org/10.1105/tpc.010378
Shimosato H, Yokota N, Shiba H et al (2007) Characterization of the SP11/SCR high-affinity binding site involved in self/nonself recognition in Brassica self-incompatibility. Plant Cell 19:107–117. https://doi.org/10.1105/tpc.105.038869
Stein JC, Howlett B, Boyes DC, Nasrallah ME, Nasrallah JB (1991) Molecular cloning of a putative receptor protein kinase gene encoded at the self-incompatibility locus of Brassica oleracea. Proc Natl Acad Sci USA 88:8816–8820. https://doi.org/10.1073/pnas88.19.8816
Suzuki G, Kai N, Hirose T, Fukui K, Nishio T, Takayama S, Isogai A, Hinata WM (1999) Genomic organization of the S locus: Identification and characterization of genes in SLG/SRK region of S9 haplotype of Brassica campestris (syn. rapa). Genetics 153:391–400
Takada Y, Murase K, Shimosato-Asano H et al (2017) Duplicated pollen-pistil recognition loci control intraspecific unilateral incompatibility in Brassica rapa. Nat Plants 3:17096. https://doi.org/10.1038/nplants.2017.96
Takada Y, Mihara A, He Y, Xie H, Ozaki Y, Nishida H, Hong S, Lim YP, Takayama S, Suzuki G, Watanabe M (2021) Genetic diversity of genes controlling unilateral incompatibility in Japanese cultivar of Chinese cabbage. Plants 10:2467. https://doi.org/10.3390/plants10112467
Takada Y, Sato T, Suzuki G, Shiba H, Takayama S, Watanabe M (2013) Involvement of MLPK pathway in intraspecies unilateral incompatibility regulated by a single locus with stigma and pollen factors. G3 Genes Genomes Genet 3: 719–726
Takasaki T, Hatakeyama K, Watanabe M, Toriyama K, Isogai A, Hinata K (1999) Introduction of SLG (S locus glycoprotein) alters the phenotype of endogenous S haplotype, but confers no new S haplotype specificity in Brassica rapa L. Plant Mol Biol 40:659–668. https://doi.org/10.1023/A:1006274525421
Takasaki T, Hatakeyama K, Suzuki G, Watanabe M, Isogai A, Hinata K (2000) The S receptor kinase determines self-incompatibility in Brassica stigma. Nature 403:913–916. https://doi.org/10.1038/35002628
Takayama S, Shiba H, Iwano M, Shimosato H, Che F-S, Kai N, Watanabe M, Hinata SG, K, Isogai A, (2000) The pollen determinant of self-incompatibility in Brassica campestris. Proc Natl Acad Sci USA 97:1920–1925. https://doi.org/10.1073/pnas.040556397
Takayama S, Shimosato H, Shiba H, Funato M, Che F-S, Watanabe M, Iwano M, Isogai A (2001) Direct ligand-receptor complex interaction controls Brassica self-incompatibility. Nature 413:534–538. https://doi.org/10.1038/35097104
Tarutani Y, Shiba H, Iwano M, Kakizaki T, Suzuki G, Watanabe M, Isogai A, Takayama S (2010) Trans-acting small RNA determines dominance relationships in Brassica self-incompatibility. Nature 466:983–986. https://doi.org/10.1038/nature09308
Tsuchimatsu T, Suwabe K, Shimizu-Inatsugi R et al (2010) Evolution of self-compatibility in Arabidopsis by a mutation in the male specificity gene. Nature 464:1342–1346. https://doi.org/10.1038/nature08927
Watanabe M, Ito A, Takada Y et al (2000) Highly divergent sequences of the pollen self-incompatibility (S) gene in class-I S haplotypes of Brassica campestris (syn. rapa) L. FEBS Lett 473:139–144. https://doi.org/10.1016/S0014-5793(00)01514-3
Watanabe M, Suwabe K, Suzuki G (2012) Molecular genetics, physiology and biology of self-incompatibility in Brassicaceae. Proc Jpn Acad Ser B 88:519–535. https://doi.org/10.2183/pjab.88.519
Yasuda S, Wada Y, Kakizaki T et al (2016) A complex dominance hierarchy is controlled by polymorphism of small RNAs and their targets. Nat Plants 3:16206. https://doi.org/10.1038/nplants.2016.206
Zhang T, Zhou G, Goring DR et al (2019) Generation of transgenic self-incompatible Arabidopsis thaliana shows a genus-specific preference for self-incompatibility genes. Plants 8:570. https://doi.org/10.3390/plants8120570
Acknowledgements
The authors thank Hiromi Masuko-Suzuki, Kana Ito, Tai Takemoto, Misono Sasaki, Toko Kanomata, and Yuta Takahashi (Tohoku University). This work was supported in part by MEXT KAKENHI (Grant Numbers JP22H02162 and JP22H05179 to M. W.), JSPS KAKENHI (Grant Numbers JP21H02162 and JP18KT0048 to M. W.; JP21H04711, JP21H05030 to S. T.; JP9K05963 and JP22K05581 to Y. T.; JP20K05982 to G. S.; JP20K05824 to K. M.).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Consent to participate
All the co-authors gave their approval to be included in the manuscript.
Consent for publication
All co-authors gave their consent for publishing this work in Plant Reproduction.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ohata, M., Takada, Y., Sato, Y. et al. MLPK function is not required for self-incompatibility in the S29 haplotype of Brassica rapa L.. Plant Reprod 36, 255–262 (2023). https://doi.org/10.1007/s00497-023-00463-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00497-023-00463-w